background and overview[1]
α-methylbenzonitrile, also called 2-phenylpropionitrile, can be used to synthesize 2-phenylpropionic acid, which is widely used in the synthesis of medicines, spices and pesticides.
preparation[1]
currently, the industrial production of α-methylbenzonitrile is mainly produced through the methylation reaction of phenylacetonitrile.
the literature (synthesis, 2018, 50(15): 2878-2886.) used phenylacetonitrile as raw material, methyl iodide as methylating reagent, and synthesized α-methylbenzene under the catalysis of lithium diisopropylamide. nitrile.
the literature (chinese journal of pharmaceutical industry, 1991 (1): 2-4.) uses dimethyl sulfate as the methylation reagent, and reacts at room temperature under the catalysis of sodium hydroxide and tetrabutylammonium bromide. in 4h, α-methylbenzonitrile was generated with a yield of 72%.
the literature (organic syntheses. john wiley & sons, inc. 2003: 169-175.) uses dimethyl carbonate as the methylating reagent, and combines phenylacetonitrile, dimethyl carbonate, and potassium carbonate in a molar ratio of 1:16.4:2.1 after mixing, the mixture was heated to 180°c in an autoclave and reacted for 18 hours to obtain α-methylbenzonitrile with a yield of 93%.
cn201811310926.5 provides a phenylacetonitrile methylation method based on pressurized distillation technology, including the following steps:
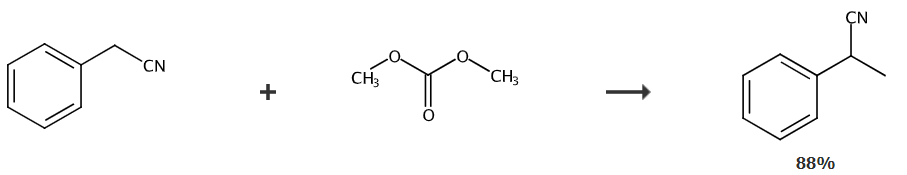
1). mix phenylacetonitrile, sodium alkoxide and dimethyl carbonate (dimethyl carbonate also serves as methylation reagent and solvent) according to the molar ratio of 1:0.1~0.4:4~10, and mix at 2± 0.2mpa pressure, 160~200℃ reaction temperature, 4~8h reaction;
2) after the reaction is completed, the temperature is lowered (100±5°c) to normal pressure distillation, then vacuum distillation (20mmhg), and the fractions between 108 and 113°c are collected to obtain α-methylbenzonitrile as the product .
apply[2]
α-methylbenzonitrile can be used to prepare 2-phenylpropionic acid. cn201410527921.3 reports a synthesis method of 2-phenylpropionic acid, which belongs to the field of medicine. α-methyl benzonitrile undergoes alkaline hydrolysis and acidification to obtain 2-phenylpropionic acid. the crude product is distilled under reduced pressure of 3 mmhg to obtain high-purity 2-phenylpropionic acid with an hplc purity greater than 99%. the use of precious metal complexes is avoided during the entire reaction process, and the process is simple and easy to operate.
main reference materials
[1] [chinese invention] cn201811310926.5 methylation method of phenylacetonitrile based on pressure distillation technology
[2]cn201410527921.3 a kind of preparation method of 2-phenylpropionic acid

 微信扫一扫打赏
微信扫一扫打赏

