background and overview[1]
o-fluoroanisole can be used as a pharmaceutical synthesis intermediate. if o-fluoroanisole is inhaled, please move the patient to fresh air; if the skin comes into contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical treatment if you feel uncomfortable; if the eye contact occurs, seek medical attention. separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
preparation[1]
the preparation of o-fluoroanisole is as follows:

the specific steps are as follows: dissolve o-fluorophenol (4.0g, 35.7mmol) in tetrahydrofuran (30ml), add potassium carbonate (18.5g, 0.13mol) and dimethyl sulfate (4.2ml, 44.3mmol), obviously the reaction was exothermic at 50°c for 1 hour, and tlc showed that the raw materials had disappeared. filter off potassium carbonate, wash the solid with ethyl acetate (3×20ml), combine the organic phases, dry over anhydrous magnesium sulfate, and concentrate under reduced pressure (water pump) to obtain light yellow oily liquid o-fluoroanisole (3.74g, yield 83%). δh(300mhz; cdcl3)7.07-6.84(4h, m), 3.87(3h, s).
application
o-fluoroanisole can be used as a pharmaceutical synthesis intermediate. if the following reaction occurs:
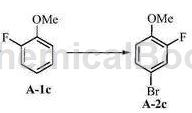
the specific steps are as follows: dissolve the crude product of o-fluoroanisole (1.0g, 7.9mmol) in acetonitrile (15ml), add n-bromosuccinimide (1.4g, 8.7mmol), and stir at 70 react for 3 hours at ℃. tlc shows that the raw materials have been reacted. most of the acetonitrile is evaporated, 30 ml of water is added, stirred for 10 min, extracted with ethyl acetate (3×20 ml), the organic phases are combined, dried over anhydrous magnesium sulfate and subjected to column chromatography. after separation (pure petroleum ether was used as eluent), the brominated product was obtained as a light yellow oily liquid (1.4 g, yield 86%). a-2c: δh (300mhz; cdcl3) 7.23-7.16 (2h, m), 6.82 (1h, t, j=8.7hz), 3.86 (3h , s); δc (75mhz; cdcl3)152.3 (d, jc, f=249.0hz), 147.1 (d, j=10.4hz), 127.2 (d, j=4.0hz), 119.6 (d, j=21.1hz), 114.6 (d, j=2.2hz), 111.9 (d, j=8.2hz), 56.4.
main reference materials
[1] cn201410820897.2 monofluorinated radicamine compound and its application and preparation method

 微信扫一扫打赏
微信扫一扫打赏

