background and overview[1]
heterocyclic compounds are widely found in nature, accounting for about half of organic compounds. 2-aminothiazole ring derivatives are a very important member of heterocyclic compounds. 2-amino-4-(3-bromophenyl)thiazole can be used as a pharmaceutical synthesis intermediate. this structure is widely used in the fields of pesticides and medicine. many natural products or small molecule drugs with anti-inflammatory, anti-tumor, anti-viral, and sedative effects contain 2-aminothiazole rings, which have become one of the most commonly constructed functional groups in green drug research in recent years. one.
so far, a variety of methods for constructing 2-aminothiazole ring functional groups have been reported, the most classic of which is prepared by refluxing 2-bromoacetophenone with thiourea in ethanol. in addition, benzene formyl isothiocyanates, secondary amines, and butynyl esters use enzyme-catalyzed one-pot methods to construct this functional group, as well as nanoparticles and iodine-catalyzed conversion of acetophenone into thiazole rings. however, these methods generally have complex operations and reaction problems. disadvantages include severe conditions and difficulty in control. some studies have developed a method for synthesizing 2-aminothiazole ring compounds from ethylbenzene compounds with high yield, mild reaction, low cost and environmental friendliness.
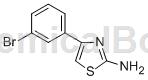
preparation[1]
the preparation of 2-amino-4-(3-bromophenyl)thiazole is as follows: add 3ml water, 30μl cyclodextrin, 0.50mmol 1-bromo-3-ethylbenzene, 1.50mmol nbs and 0.05mmol aibn, react at 60°c for 4 hours, cool after the reaction is completed, then add 1.50mmol sodium bicarbonate and 0.50mmol thiourea in sequence, react at 80°c for 1 hour, add ethyl acetate after the reaction, add saturated brine for extraction, and concentrate the organic phase, column chromatography obtained 97 mg of white solid 2-amino-4-(3-bromophenyl)thiazole, with a yield of 76%.
product characterization: 1hnmr (cdcl3, 600mhz) δ: 7.97 (s, 1h), 7.72 (d, j=7.5hz, 1h), 7.44 (d, j=7.7hz, 1h), 7.3-7.26 (m , 1h), 6.78(s, 1h), 5.20(s, 2h).
main reference materials
[1] (cn108863978) a method for synthesizing 2-aminothiazole ring compounds from ethylbenzene compounds

 微信扫一扫打赏
微信扫一扫打赏

