background and overview[1]
2,4,6-tri-tert-butylnitrosobenzene can be used as a pharmaceutical synthesis intermediate. if 2,4,6-tri-tert-butyl-nitrosobenzene is inhaled, please move the patient to fresh air; if the skin comes into contact, take off contaminated clothing and wash the skin thoroughly with soap and water. if you feel discomfort , seek medical attention; if eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
preparation[1]
the preparation of 2,4,6-tri-tert-butyronitrosobenzene is as follows:
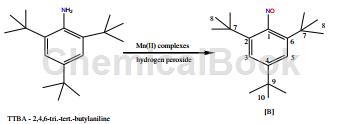
dissolve ttba (2,4,6-tri-tert-butylaniline, 127mm) in 3 ml meoh, add [mn (l) cl2] complex (12.7mm) to the suspension in meoh, and then add h2o2 (127mm of 2,4,6-tri-tert-butylaniline). the above reaction was repeated using tbhp instead of h2o2 as the oxidant (2,4,6-tri-tert-butylaniline was 127mm). the reaction mixture was stirred in a water bath at a temperature of 45°c for 48 hours and monitored continuously using tlc. 2 spots were observed at different rf values and the reaction mixture was run on a short column after 48 hours and eluted with 20% ethyl acetate/hexane. evaporation of the eluate to a small volume and holding overnight gave the desired product 2,4,6-tri-tert-butyronitrosobenzene. the above oxidation reaction can also use [mn(l)(ch3coo)2] as a catalyst instead of [mn(l)cl2], but the product distribution obtained by using the [mn(l)(ch3coo)2] complex as a catalyst is very complex, and the column chromatography did not produce the separation of a single product. this may also explain why there are many products using [mn(l)(ch3coo)2] as the oxidation catalyst to obtain hindered phenols and anilines.
1hnmr(d6-dmso)(ppm):1.22(s,9h),1.38(s,18h),7.06(s,2h).13cnmr(d6-dmso):168.0(c-1),142.1( c-4), 133.0 (c-2, c-6), 121.3 (c-3, c-5), 34.8 (c-7), 30.5 (c-8), 34.3 (c9), 32.0 (c- 10).esims+(m/e):274,261,246,230.ir(cm1):1620,1363,%yield:75(yieldsrefertoisolatedyield).
main reference materials
[1] organo-peroxyl compounds via catalytic oxidation of a hindered phenol and
aniline utilizing new manganese(ii) bis benzimidazole diamide based complexes

 微信扫一扫打赏
微信扫一扫打赏

