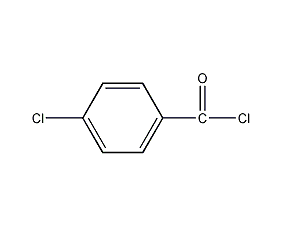
structural formula
| business number | 03ee |
|---|---|
| molecular formula | c7h4cl2o |
| molecular weight | 175 |
| label |
p-chlorobenzoyl chloride, p-chlorobenzoyl chloride, 4-chlorobenzoyl chloride, p-chlorobenzoyl chloride, 4-chlorobenzoyl chloride, 4-chlorobenzoylamino, 4-chloro-benzoylchlorid, benzoyl chloride, 4-chloro-, benzoyl chloride, p-chloro- |
numbering system
cas number:122-01-0
mdl number:mfcd00000686
einecs number:204-515-3
rtecs number:dm6635510
brn number:471606
pubchem id:none
physical property data
1. properties: colorless or slightly yellow liquid
2. density (g/ml, 25/4℃): 1.374~1.376
3. refractive index (nd20): 1.5780
4. flash point (℃): 105
5. freezing point (℃): 12-14
6. boiling point (ºc): 220~222
7. solubility: insoluble in water, soluble in alcohol, ether and acetone. decomposes in water.
toxicological data
none
ecological data
none
molecular structure data
1. molar refractive index: 41.39
2. molar volume (cm3/mol): 127.7
3. isotonic specific volume (90.2k): 326.9
4. surface tension (dyne/cm): 42.8
5. dielectric constant:
6. dipole moment (10-24 cm3):
7. polarizability: 16.40
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 0
3. number of hydrogen bond acceptors: 1
4. number of rotatable chemical bonds: 1
5. number of tautomers: none
6. topological molecule polar surface area 17.1
7. number of heavy atoms: 10
8. surface charge: 0
9. complexity: 128
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
this product can irritate mucous membranes, cause tears, and corrode the skin.
�how to save
stored in a cool, ventilated and dry warehouse.
it is a first-level organic acid corrosive substance.
synthesis method
obtained from the reaction of p-chlorobenzoic acid and thionyl chloride: slowly add thionyl chloride to p-chlorobenzoic acid, heat to reflux, and react at 75-85°c for 3-4 hours. then the thionyl chloride is evaporated to obtain the crude product. finally, fractions around 220°c are collected by fractionation, and then decolorized to obtain the finished product.
purpose
intermediates of dyes and drugs, used in the production of indomethacin and fenobel, etc.

 微信扫一扫打赏
微信扫一扫打赏

