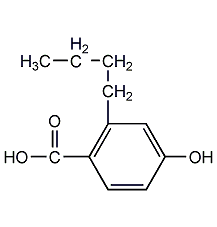
structural formula
| business number | 027g |
|---|---|
| molecular formula | c11h14o3 |
| molecular weight | 194.23 |
| label |
butylparaben, n-butyl 4-hydroxybenzoate, n-butylparaben, butylparaben, 4-hydroxybenzoicaicdbutylester, p-hydroxybenzoic acid n-butyl ester, butyl paraben, hoc6h4co2(ch2)3ch3, preservative |
numbering system
cas number:94-26-8
mdl number:mfcd00016478
einecs number:202-318-7
rtecs number:dh1980000
brn number:1103741
pubchem number:24895855
physical property data
1. properties: white crystalline powder. slightly special smell.
2. density (g/ml, 25/4℃): undetermined
3. relative vapor density (g/ml, air=1): undetermined
4. melting point (ºc): 68-69
5. boiling point (ºc, normal pressure): undetermined
6. boiling point (ºc, 5.2kpa): undetermined
7. refractive index: undetermined
8. flash point (ºc): undetermined
9. specific rotation (º): undetermined determined
10. autoignition point or ignition temperature (ºc): not determined
11. vapor pressure (kpa, 25ºc): not determined
12. saturated vapor pressure (kpa, 60ºc): undetermined
13. heat of combustion (kj/mol): undetermined
14. critical temperature (ºc): undetermined
15. critical pressure (kpa): undetermined
16. log value of oil-water (octanol/water) partition coefficient: undetermined
17. explosion upper limit ( %, v/v): undetermined
18. lower explosion limit (%, v/v): undetermined
19. solubility: soluble in ethanol, acetone, chloroform and diethyl ether, slightly soluble in water and propylene glycol.
toxicological data
none yet
ecological data
molecular structure data
1. molar refractive index: 53.87
2. molar volume (cm3/mol): 166.2
3. isotonic specific volume (90.2k): 440.5
4. surface tension (dyne/cm): 49.3
5. polarizability (10-24cm3): 21.35
calculate chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 1
3. number of hydrogen bond acceptors: 3
4. number of rotatable chemical bonds: 5
5. number of tautomers: 4
6. topological molecule polar surface area 46.5
7. number of heavy atoms: 14
8. surface charge: 0
9. complexity: 171
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
none yet
storage method
store in a sealed, cool and dry place.
synthesis method
1. obtained from the esterification of p-hydroxybenzoic acid and butanol. heat butanol and p-hydroxybenzoic acid together to dissolve, slowly add sulfuric acid dropwise, and continue the reflux reaction 8h after the addition is complete. let cool, add 4% sodium carbonate solution, separate the water layer, drive out the butanol with steam, let cool, filter to get the crude product, and then recrystallize with ethanol (in solubility in ethanol: 200g/100ml).
purpose
1. antiseptics and disinfectants.
2. organic synthesis intermediates; antiseptic additives for medicine, food, cosmetics, film and high-end products; reagents. its main use is as a preservative and bactericidal agent for food. among parabens, it has the strongest antiseptic and bactericidal power. because of its poor water solubility, it is often used together with other esters.

 微信扫一扫打赏
微信扫一扫打赏

