background and overview[1]
3,3-dichloro-1-(4-nitrophenyl)-2-piperidone is an important pharmaceutical intermediate, for example, it can be used to prepare apixaban derivatives. apixaban is a small molecule selective fxa inhibitor of pyrazole derivatives. similar to rivaroxaban, apixaban has two binding sites for factor xa. the 4-methoxyphenyl moiety in the apixaban structure binds to the s1 pocket of factor xa, and the aryllactam moiety binds to factor xa. factor s4 pocket. apixaban is a highly selective inhibitor of factor thrombin is not affected, and apixaban selectively inhibits factor xa 30,000 times more than other thrombins.
preparation[1]
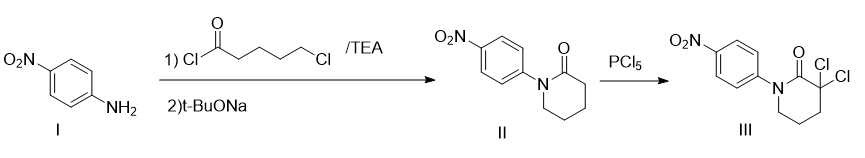
synthesis of 1-(4-nitrophenyl)piperidin-2-one (ii)
add p-nitroaniline (40g, 0.28mol), dry tetrahydrofuran (600ml), and triethylamine (60ml, 0.44mol) into a 1000ml three-neck flask. under mechanical stirring, add 5-chlorovaleryl chloride in batches. (56ml, 0.44mol), after addition, react at 60°c for 2h. then the temperature was lowered to -10°c, and sodium tert-butoxide (69.8g, 0.92mol) was added in batches. during the addition process, the temperature was controlled below 0°c. after the addition, the temperature was raised to 50°c and the reaction was carried out for 6 hours. tetrahydrofuran was evaporated under reduced pressure, and 500 ml of saturated sodium carbonate aqueous solution was added to the residue for slurry washing. a large amount of solid precipitated. the product was obtained by suction filtration, an earthy yellow solid, 52.6 g, m.p. 96-99°c, yield 85.4%.
synthesis of 3,3-dichloro-1-(4-nitrophenyl)-2-piperidone (iii)
add compound ii (40g, 0.18mol) and chlorobenzene (400ml) into a 1000ml eggplant-shaped flask. add phosphorus pentachloride (132.4g, 0.64mol) in batches while stirring. after adding, raise the temperature to 55°c for reaction. 5h. cool the reaction solution to room temperature, pour into 1000 ml of ice water, separate the lower layer, and extract with 3 × 200 ml of methylene chloride. combine the organic phases, wash twice with 2 × 200 ml of water, once with 400 ml of saturated brine, and wash with anhydrous sodium sulfate. dry and evaporate to dryness in methylene chloride to obtain the product, a light yellow solid, 49.4g, m.p. 115-117°c, yield 94.9%.
apply[1]
3,3-dichloro-1-(4-nitrophenyl)-2-piperidone can be used in 3-morpholinyl-1-(4-nitrophenyl)-5,6-di synthesis of hydropyridin-2(1h)-one (iv): add 3,3-dichloro-1-(4-nitrophenyl)-2-piperidone (40g, 0.14mol) into a 500ml eggplant-shaped bottle ), morpholine (160ml, 1.84mol), heated to 130°c and reacted for 5h. recover morpholine under reduced pressure, add 400 ml of water to the residue, stir for 30 minutes, filter with suction, and dry to obtain 57.8 g of crude product. recrystallize it 8 times with ethyl acetate to obtain the product, a light yellow solid, 27.2 g, m.p. 158-160°c. yield 64.5%.
main reference materials
[1]cn201710733736.3 apixaban derivatives and preparation methods and uses

 微信扫一扫打赏
微信扫一扫打赏

