background and overview[1]
1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-4,5,6,7-tetra hydro-1h-pyrazolo[3,4-c]pyridine-3-carboxylic acid ethyl ester is an intermediate for the preparation of apixaban, an antithrombotic drug produced by bristol-myers squibb in the united states. -the factor xa direct inhibitor jointly developed by bristol-myers squibb and pfizer was approved for marketing in the eu in march 2011, and the fda approved the drug for marketing in the united states in december 2012.
preparation[1]
1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-4,5,6,7-tetra hydrogen-1h-pyrazolo[3,4-c]pyridine-3-carboxylic acid ethyl ester is prepared as follows:
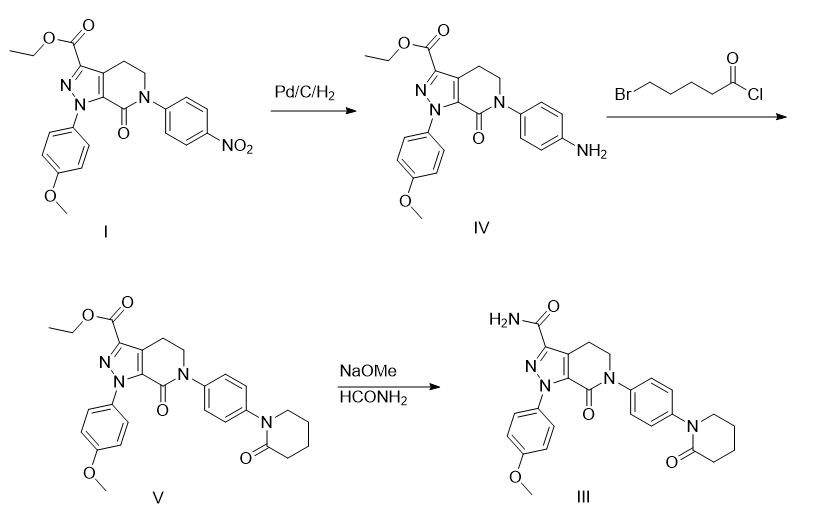
1) add 16.9g of apixaban intermediate i to the hydrogenation kettle, with a mass percentage of 5% palladium on carbon catalyst (the mass percentage refers to the mass of palladium as a percentage of the total mass of palladium on carbon )1.7g, ethanol 170ml, stir at 0.3~0.4mpa hydrogen pressure and 50~55°c for 4 hours. cooling, filtering, washing and vacuum concentration (45°c ~ 55°c, -0.085mpa ~ -0.1mpa) yielded 15.7g of apixaban intermediate iv, with a yield of 100%.
2) dissolve 15.7g of apixaban intermediate iv in 160ml of anhydrous dichloromethane, add 4.0g of finely ground sodium hydroxide, dropwise add 8.4g of 5-bromovaleryl chloride, and then add the ground add 4.0g of fine sodium hydroxide and stir at 30-35°c for 6 hours. add water to quench, then extract with dichloromethane. the organic phase is washed with a mass percentage of 10% sodium bicarbonate aqueous solution (the mass percentage refers to the mass of sodium bicarbonate in the total mass of the sodium bicarbonate aqueous solution) and a mass percentage of 15% salt water. (the mass percentage refers to the mass of sodium chloride as a percentage of the total mass of the saline solution), and dried with anhydrous sodium sulfate. filter and concentrate in vacuum (25°c to 45°c, -0.075mpa to -0.09mpa), add 96 ml of ethyl acetate to the residue, heat to 75°c to dissolve, then slowly cool to 15°c to 20°c and stir for 1 hour. filter, wash with ethyl acetate, drain and dry under vacuum at -0.01mpa~-0.1mpa, 45°c~55°c for 8 hours to 12 hours, to obtain 9.52g of apixaban intermediate v, with a yield of 50.4%.
3) dissolve 9.52g of apixaban intermediate v in 95ml of n,n-dimethylformamide, then add 9.5ml of formamide, and add it dropwise at 0°c to 5°c with a mass percentage of 30 % sodium methoxide methanol solution 27.0g (the mass percentage is the mass of sodium methoxide as a percentage of the total mass of sodium methoxide methanol solution), stir at 15°c to 20°c for 4 hours. add 190 ml of water, stir for 1 hour at 15°c to 20°c, filter, wash with methyl tert-butyl ether, drain, add 24 ml of n,n-dimethylformamide, heat to 75°c to dissolve, then slowly add methyl tert-butyl ether. 96 ml of butyl ether, cool to 5°c to 10°c and stir for 1 hour. filter, wash with methyl tert-butyl ether, drain and dry under vacuum at -0.01mpa~-0.1mpa, 45℃~55℃ for 8 hours to 12 hours, to obtain 6.29g 1-(4-methoxyphenyl)- 7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-4,5,6,7-tetrahydro-1h-pyrazolo[3,4-c]pyridine -3-carboxylic acid ethyl ester, yield 70.3%, hplc purity 99.91%, maximum single impurity 0.056%.
main reference materials
[1]cn201711444031.6 preparation method of apixaban and its intermediates

 微信扫一扫打赏
微信扫一扫打赏

