background and overview[1]
1-phenyl-2,2,2-trifluoroethylamine can be used as a pharmaceutical synthesis intermediate.
preparation[1]
the preparation of 1-phenyl-2,2,2-trifluoroethylamine is as follows:
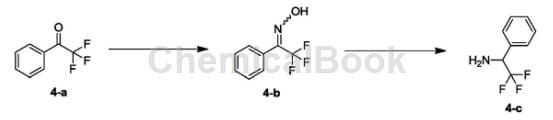
synthesis of compound 4-b:
dissolve 4-a (1.00g, 5.74mmol, 781.25ul, 1.00eq) in etoh (8.00ml) and water (4.00ml) at 25°c, then add hydroxylamine hydrochloride (598.31mg, 8.61mmol, 1.50 eq) and sodium acetate (941.70 mg, 11.48 mmol, 2.00 eq). the resulting mixture was stirred at 80°c for 15 hours. the reaction was detected by tlc (pe/ea=10/1) until the reaction was complete. the reaction solution was concentrated to remove ethanol. the obtained crude product was added with water (50 ml) and stirred at 0°c for 30 minutes, filtered and concentrated to obtain white solid compound 4-b. h-nmr (400mhz, dmso-d6) δppm7.22-7.75 (m, 5h) 12.73 (brs, 1h).
synthesis of compound 1-phenyl-2,2,2-trifluoroethylamine: dissolve 4-b (870.00mg, 4.59mmol, 1.00eq) in etoh (20.00ml) and hoac (2.00ml) then, 10% pd/c (87.00 mg) was added under nitrogen atmosphere. the resulting suspension was replaced three times with h2 and stirred at 25°c for 15 hours in the presence of h (50 psi). tlc (pe/ea=3/1) detected the reaction until completion. the reaction solution was filtered and concentrated under reduced pressure. 1nnaoh (10 ml) was added to the crude product and extracted with ea (25 ml*2). the organic phases were combined and washed with saturated brine (50 ml*2), dried over naso4, filtered and concentrated to obtain colorless oily liquid 1-phenyl-2,2,2-trifluoroethylamine.
1hnmr (400mhz, dmso-d6) δppm4.48 (q, j=7.91hz, 1h) 7.26-7.62 (m, 5h).
application
1-phenyl-2,2,2-trifluoroethylamine can be used as a pharmaceutical synthesis intermediate. if the following reaction occurs:
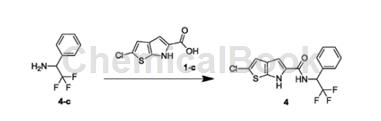
the specific steps are: combine 1-phenyl-2,2,2-trifluoroethylamine (324.99mg, 691.74umol, 1.10eq) and 1-c (150.00mg, 628.85umol, 1.00eq) at 25°c ) was dissolved in dmf (10.00ml) and then tbtu (302.87mg, 943.27umol, 1.50eq) and dipea (243.82mg, 1.89mmol, 329.48ul, 3.00eq) were added. the resulting mixture was stirred at 25°c for 12 hours. reaction completion was detected by tlc (pe/ea=3/1) and lc-ms. water (20 ml) was added to the reaction solution and extracted with ea (50 ml*3). the organic phases were combined and washed with saturated brine (30 ml*2), dried over naso4, filtered and concentrated to obtain the crude product. the crude product was purified by prep-hpl (column: bostongreenods150*305u; mobilephase: [water (0.225% fa)-acn]; b%: 55%-82%, 10min) to obtain compound 4.
hnmr (400mhz, dmso-d6) δppm6.02 (quin, j=8.91hz, 1h)7.20 (s, 1h)7.36 (s, 1h)7.39-7.50 (m, 3h)7.68 (brd, j= 6.40hz, 2h) 8.43 (s, 1h) 9.17 (brd, j = 9.29hz, 1h) 11.60-12.49 (m, 1h).
main reference materials
[1]cn201810497159.7ido inhibitor

 微信扫一扫打赏
微信扫一扫打赏

