background and overview[1]
4-(4-methylpiperazinemethyl)benzoyl chloride dihydrochloride can be used as a pharmaceutical synthesis intermediate. if 4-(4-methylpiperazinemethyl)benzoyl chloride dihydrochloride is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing and wash skin thoroughly with soap and water. , if you feel discomfort, seek medical attention; if the eye contact occurs, separate the eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse your mouth immediately, do not induce vomiting, and seek medical attention immediately.
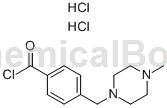
preparation[1]

put 1g of imacic acid (5g, 0.021mol, anaiji chemical) into a 100ml three-necked flask, add sulfonyl chloride (35ml) and catalyst n,n-dimethylformamide (0.3ml), and heat to reflux react for 22 hours, lower to room temperature, filter with suction, wash with n-hexane (10ml×2), and vacuum dry at 45-50°c for 2 hours to obtain white solid 4-(4-methylpiperazinemethyl)benzoyl chloride. hydrochloride (1h) 5.1g, yield 95%.
apply[1]
4-(4-methylpiperazinemethyl)benzoyl chloride dihydrochloride can undergo the following reactions:
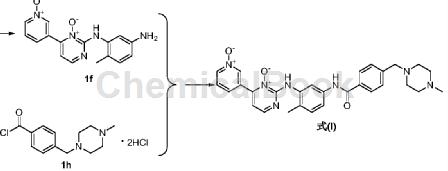
put 1f (1.83g, 0.006mol) into a 100ml three-necked flask, add purified water (31ml), stir to lower the internal temperature to 0~3°c, and add 4-(4-methylpiperazinemethyl)benzene in batches formyl chloride dihydrochloride (3.9g, 0.015mol), stir at room temperature for 2 hours, filter, add acetone (16ml) to the filtrate, heat to 45-50°c, add ammonia dropwise until the cloud point appears, cool to room temperature, stir and precipitate crystallize for 2 hours, filter with suction, wash with purified water (10ml×2), and vacuum dry at 75-80°c for 2 hours to obtain 4-[(4-methyl-1-piperazinyl)methyl]- as a yellow solid n-[4-methyl-3-[[4-(1-oxo-3-pyridyl)-3-oxo-2-pyrimidinyl]amino]phenyl]benzamide formula (i) 2.29g, collected the rate is 73%, hplc: 99.2%.
1hnmr (dmso-d6, 500mhz): δ2.17(s, 3h), δ2.30(s, 3h), δ2.35( m, 8h), δ3.55 (s, 2h), δ7.31 (d, 1h), δ7.50 (m, 4h), δ7.66 (d, 1h), δ8.00 (d, 2h), δ8.18(d, 1h), δ8.28(d, 1h), δ8.63(s, 1h), δ8.75(d, 1h), δ8.89(s, 1h), δ9.75(s , 1h), δ10.26 (s, 1h).
main reference materials
[1] cn107573322 imatinib bis-nitroxide, its preparation method and use

 微信扫一扫打赏
微信扫一扫打赏

