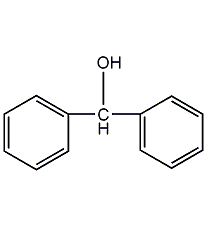
structural formula
| business number | 022z |
|---|---|
| molecular formula | c13h12o |
| molecular weight | 184.23 |
| label |
benzyl alcohol, α-phenylbenzyl alcohol, diphenylmethanol, diphenyl carbinol, (c6h5)2choh |
numbering system
cas number:91-01-0
mdl number:mfcd00004488
einecs number:202-033-8
rtecs number:dc7452000
brn number:1424379
pubchem number:24866820
physical property data
1. properties: colorless needle-like crystals.
2. density (g/ml, 25/4℃): undetermined
3. relative vapor density (g/ml, air=1): undetermined
4. melting point (ºc): 69
5. boiling point (ºc, normal pressure): undetermined
6. boiling point (ºc, 99.84kpa): 298
7. refractive index: undetermined
8. flash point (ºc): undetermined
9. specific rotation (º): undetermined
p>
10. autoignition point or ignition temperature (ºc): undetermined
11. vapor pressure (kpa, 25ºc): undetermined
12. saturated vapor pressure (kpa, 60ºc): undetermined
13. heat of combustion (kj/mol): undetermined
14. critical temperature (ºc): undetermined
15. critical pressure (kpa): undetermined
16. log value of oil-water (octanol/water) partition coefficient: undetermined
17. explosion upper limit (%, v /v): undetermined
18. lower explosion limit (%, v/v): undetermined
19. solubility: easily soluble in ethanol, ether, chloroform and carbon disulfide, at 20°c, 1g of product is soluble in 2000ml of water and almost insoluble in cold crude gasoline.
toxicological data
acute toxicity:
oral ld50 5mg/kg(rat)
skin ld50 >5mg/kg(rbt)
skin irritation mild unknown mg ( rbt)
eye irritation mild unknown mg (rbt)
main irritating effects:
on skin: may cause inflammation
on eyes: may cause inflammation
sensitization: may cause sensitization through skin contact
ecological data
general remarks
water hazard class 1 (german regulations) (self-assessment via list) the substance is slightly hazardous to water.
do not allow undiluted or large amounts of product to come into contact with groundwater, waterways or sewage systems.��
do not discharge materials into the surrounding environment without government permission.
molecular structure data
1. molar refractive index: 57.10
2. molar volume (cm3/mol): 167.0
3. isotonic specific volume (90.2k ): 433.7
4. surface tension (dyne/cm): 45.3
5. polarizability (10-24cm3): 22.63
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 1
3. number of hydrogen bond acceptors: 1
4. number of rotatable chemical bonds: 2
5. number of tautomers: none
6. topological molecule polar surface area 20.2
7. number of heavy atoms: 14
8. surface charge: 0
9. complexity: 137
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
this product should be sealed and stored in a cool place.
storage method
none
synthesis method
1. preparation method:
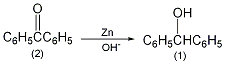
in a 1l reaction bottle equipped with a stirrer, thermometer and reflux condenser, add 50g of benzophenone (2) (0.275mopl), 500ml (95%) of ethanol, 50g of sodium hydroxide and 50g of zinc powder (0.76mol ), stir vigorously, and slowly raise the temperature to about 70°c. after 3 hours, lower the temperature, filter, and wash the filter residue twice with hot 95% ethanol (the remaining zinc powder cannot become dry to avoid catching fire). pour the filtrate into 2l ice water and acidify it with 100ml concentrated hydrochloric acid to precipitate a white solid. after suction filtration and drying in the air, 49g of crude product benzyl alcohol was obtained, mp65°c. recrystallize with 50 ml of hot ethanol, cool in an ice-salt bath, filter the precipitated colorless solid, and dry to obtain pure benzyl alcohol ①(1) 36g, mp68°c. some products can be recovered from the mother liquor. note: ① benzophenone can also be prepared by reducing benzophenone with sodium borohydride in 95% ethanol, with a yield of about 90%. [1]
purpose
used in organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

