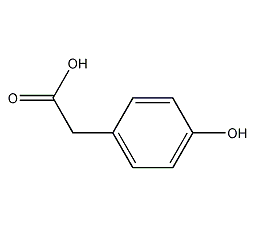
structural formula
| business number | 03zk |
|---|---|
| molecular formula | c9h8o3 |
| molecular weight | 152 |
| label |
4-hydroxyphenylacetic acid, 4-hydroxyphenylacetic acid, 4-hydroxybenzeneacetic acid, pesticide intermediates; aromatic carboxylic acids and their derivatives, acidic solvent |
numbering system
cas number:156-38-7
mdl number:mfcd00004347
einecs number:205-851-3
rtecs number:ai2680000
brn number:1448766
pubchem id:none
physical property data
1. properties: white crystalline powder.
2. melting point (ºc): 149-150
3. solubility: slightly soluble in water, soluble in ether, ethanol, ethyl acetate .
4. boiling point (ºc): 346.6
5. flash point (ºc): 177.6
6. relative density (d20
sup>4) :1.319
toxicological data
on skin: irritation to skin and mucous membranes
on eyes: irritating effects
sensitization: no known sensitizing effects
ecological data
none
molecular structure data
1. molar refractive index: 39.24
2. molar volume (cm3/mol): 115.3
3. isotonic specific volume (90.2k ): 320.6
4. surface tension (dyne/cm): 59.8
5. dielectric constant: not available
6. dipole moment (10 -24cm3): not available
7. polarizability: 15.55
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 2
3. number of hydrogen bond acceptors: 3
4. number of rotatable chemical bonds: 2
5. number of tautomers: 2
6. topological molecule polar surface area 57.5
7. number of heavy atoms: 11
8. surface charge: 0
9. complexity: 136
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
1. materials to avoid: oxides. hazardous decomposition products: carbon monoxide and carbon dioxide.
2. exist in flue-cured tobacco leaves, burley tobacco leaves, oriental tobacco leaves and smoke.
storage method
store in an airtight container and in a cool, dry place.
synthesis method
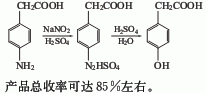
1.it is obtained by diazotization and hydrolysis of p-aminophenylacetic acid. prepare sodium salt of p-aminophenylacetic acid and alkali solution, then add sulfuric acid. cool to 0℃, control the temperature at 0-5℃, add sodium nitrate solution dropwise, and complete the reaction for 0.5h. . add the obtained diazo liquid dropwise to the dilute sulfuric acid at 90-95°c, complete the dripping in about 1 hour, and then continue the reaction for 1 hour. the reaction solution is decolorized, filtered, cooled and extracted with ethyl acetate. the finished product is obtained after recovering ethyl acetate from the extract. the yield is about 85%.
2.3-fluoro-p-hydroxyphenylacetic acid
2. tobacco: or, 41 ; fc, 54; bu, 26.
purpose
organic synthesis intermediate, used in the production of β-receptor blocker atenolol and the synthesis of 4,7-dihydroxyisoflavone, the active ingredient of kudzu daidzein; it can also be used as a pesticide intermediate.

 微信扫一扫打赏
微信扫一扫打赏

