background and overview[1]
for the currently known reported 4-aminobenzene borate hydrochloride, 4-aminobromobenzene is usually used as the raw material. after the amino group is protected, n-butyllithium and borate are used at low temperature conditions of around -78°c. after reaction, the product is obtained after acidification and purification. this method has the following disadvantages: 1. the raw materials need to be protected and deprotected by amino groups, which is cumbersome to operate; 2. the reaction requires ultra-low temperature -78°c and the conditions are harsh.
preparation method[1]
a method for preparing 4-aminophenyl borate hydrochloride using biscatechol borate:
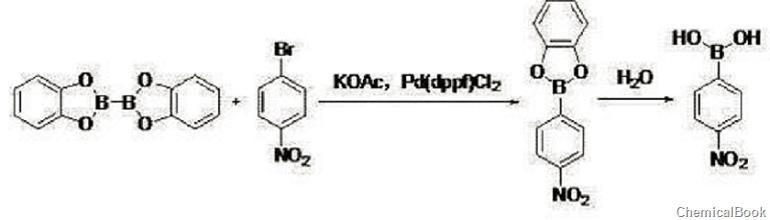
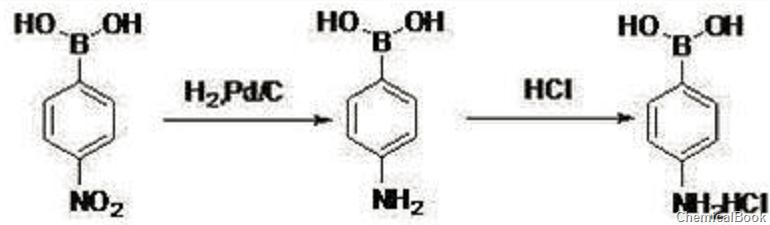
the specific steps are as follows:
in the first step, add 500.0g dmf, 101.0g 4-nitrobromobenzene (0.50mol, 1eq), and 130.8g dicatecholboric acid into a 1l four-necked flask with magnetic stirring under nitrogen protection. ester (0.55mol, 1.2eq), 147.2g potassium acetate (1.5mol, 3eq), 18.3gpd(dppf)cl2 (0.025mol, 0.05eq), react at 80°c for 3h. lower the temperature to 20~25°c, filter, add the filtrate dropwise to 500.0g of water, stir for 1 hour, and raise the temperature to 20~25°c. extract twice with ethyl acetate (200 ml %.
in the second step, dissolve 63.7g 4-nitrobenzene boric acid (0.38mol, 1eq) into 255g ethyl acetate, add 2.0g palladium carbon (10%, 0.005eq), 0.8~1.0mpa, 70~80 ℃ hydrogenation reaction for 6 hours, the reaction is completed, use diatomaceous earth to filter, the filtrate is cooled to 0℃, dropwise add 51.0g concentrated hydrochloric acid (30%, 0.38mol, 1.1eq), stir at 0~10℃ for 0.5h, filter, filter cake use 80g acetone to beat to obtain 52.4g off-white solid 4-aminobenzene borate hydrochloride, hplc: 98.2%, the second step yield is 79.2%, and the total yield is 60.4%.
main reference materials
[1] cn201210557943.5 method for preparing 4-aminobenzene borate hydrochloride

 微信扫一扫打赏
微信扫一扫打赏

