background and overview[1]
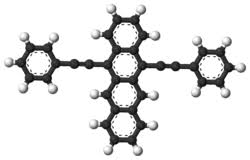
5,12-bis(phenylethynyl)tetracene is an organic synthesis intermediate and pharmaceutical intermediate, which can be used in laboratory research and development processes and chemical and pharmaceutical research and development processes.
preparation[1]
5,12-bis(phenylethynyl)tetracene is prepared as follows:
1) add 51.0g (0.50 mol) anhydrous dioxane to a suspension of 11.5g (0.50 mol) lithium amide in 600 ml, and heat the mixture to reflux for 2 hours. to the warm mixture was added 52.0 g (0.50 mol) of 9,10-anthraquinone, and the mixture was heated to reflux for 16 hours. the cooled mixture was treated with 1 liter of 0.5m aqueous ammonium chloride solution. the product was filtered off and washed with water. recrystallization from acetonitrile gave colorless crystals. 220°c (melting point m.p. 206-207), yield 47%.
2) dissolve 5 grams of 9,10-dihydroxy-9,10-bis(phenylethynyl)9,10-dihydroanthracene in 50 ml of dioxin.
3) prepare lithium phenylacetylene from 14.85 g (0.146 mol) of phenylacetylene and 2.80 g (0.122 mol) of lithium amide in 100 ml, and then add 15.7 g of 0.25 (0.061) to 150 ml of 0.83 dioxane mol) of 5,12-naphthoquinone and the mixture was refluxed for 4 hours, then cooled and treated with 3.50 ml of 0.5 m aqueous ammonium chloride solution. the product was recrystallized from benzene to obtain 15.95 g (57%) of colorless crystals of 5,12-dihydroxy-5,12-bis(phenylethynyl)-5,12-dihydronaphthocene, m.p. 216.5-218℃ (decomposition).
4) in 200 ml of 29 g of stannous chloride solution, slowly add 14.4 g of 5,12-dihydroxy-5,12-bis-(phenylethynyl)-5,12-di in 50 ml of acetic acid aqueous solution. hydronaphthalene, incorporated into dioxane. the mixture was stirred at room temperature for 2 hours, then diluted to a volume of 1,500 ml, and the crude product was recrystallized from benzene to obtain 8.1 g (63%) of dark red-purple needle 5,12-bis(phenylacetynyl)tetracene.
main reference materials
[1] us3557233

 微信扫一扫打赏
微信扫一扫打赏

