background and overview[1]
3-bromo-1,3,4,5-tetrahydro-2h-1-benzazepine-2-one is an intermediate in the synthesis of benazepril hydrochloride. benazepril hydrochloride is a first-line antihypertensive drug recommended by the world health organization. it is a chiral drug with good clinical efficacy, high safety and few side effects among the current international angiotensin-converting enzyme inhibitor antihypertensive drugs. .
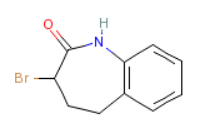
3-bromo-1,3,4,5-tetrahydro-2h-1-benzazepine-2-one
preparation[2]
the synthesis methods reported in the literature mainly include: 1. using a composite catalyst of chromium acetate and 2-methyl-5-ethylpyridine, using air as the oxidant, performing normal pressure continuous liquid phase oxidation, the reaction temperature is 130°c, and tetrahydrogen the molar ratio of naphthalene to oxygen is 1:0.6, and the residence time is 60-70 minutes. the selectivity of generating 1-tetralone is about 90%, and the main by-product is tetralone. the product is subjected to bromination, oximation and rearrangement to obtain 3-bromo-1,3,4,5-tetrahydro-2h-1-benzazepine-2-one 2.γ-benzenebutyric acid cyclization method: γ-phenylbutyric acid is cyclized under the action of phosphoric acid/phosphoric anhydride, polyphosphoric acid, hydrofluoric acid or concentrated sulfuric acid at 90°c to obtain 1-tetralone. after bromination, oximation and rearrangement, 3-tetralone is obtained. bromo-1,3,4,5-tetrahydro-2h-1-benzazepine-2-one. 3. γ-benzenebutyric acid chloride cyclization method: this product is obtained by dehydrochlorination and cyclization in the presence of anhydrous aluminum trichloride or anhydrous tin tetrachloride. after bromination, oximation and rearrangement, 3-bromo-1,3,4,5-tetrahydro-2h-1-benzazepine-2-one is obtained.
the above methods mainly have the following shortcomings: 1. there are many by-products and it is inconvenient to separate the products.
2. the yield is low and the purification is complicated.
3. this route has a low yield and is not suitable for industrial production.
4. there are many reaction steps and complicated operations.
specific synthesis method:
1) α-tetralone preparation stage
add γ-butyrolactone, benzene, and anhydrous aluminum chloride into the dry reaction kettle at a molar ratio of 1:10:3. while stirring, slowly raise the temperature to 65°c and keep the reaction for 28 hours; the reaction is completed. after that, it is hydrolyzed by 25 parts by weight of 6% hydrochloric acid solution, separated into layers, and washed with water until neutral; then excess benzene is removed by distillation, and α-tetralone is obtained by high vacuum distillation;
2) preparation stage of 2-bromo-3,4-dihydro-n-hydroxy-(2h)-naphthalimine
add α-tetralone and methanol into a dry reaction kettle at a molar ratio of 1:25, and under stirring, drop bromine with a molar ratio of 1.3 at -10°c for bromination reaction; the reaction is completed add hydroxylamine sulfate with a molar ratio of 1.6, slowly raise the temperature to 38°c, add 7 parts by weight of water to dilute and keep the reaction for 85 hours; centrifuge, wash and dry to obtain 2-bromo-3,4-dihydro-n-hydroxy-( 2h)-naphthalimine;
3) 3-bromo-1,3,4,5-tetrahydro-2h-1-benzazepine-2-one stage
add 5.5 parts by weight of heated and melted polyphosphoric acid into a dry reaction kettle, and add the above-mentioned 2-bromo-3,4-dihydro-n-hydroxy-(2h)- in batches under stirring at a temperature of 95°c. naphthimine, keep warm for 5 hours; add the reaction solution dropwise to 12.5 parts by weight of water while hot for hydrolysis, centrifuge and wash to obtain 3-bromo-1,3,4,5-tetrahydro-2h-1-benzazepine crude azo-2-one; add 10 parts by weight of methanol and 0.1 parts by weight of activated carbon, heat for decolorization and filtration, then cool the filtrate to -10°c to crystallize for 3 hours, centrifuge, wash and dry to obtain 3-bromo-1,3. 4,5-tetrahydro-2h-1-benzazepine-2-one finished product. after testing, the purity of the finished product 3-bromo-1,3,4,5-tetrahydro-2h-1-benzazepine-2-one is as high as 99.5%, and the yield is as high as 97%.
main reference materials
[1] jin dacheng, zhang wei, & park fengyu. (2010). 1,3,4,5-tetrahydro-7-alkoxy-2h-1-benzazepine-2-one synthesis and biological activity. chemical bulletin (02), 94-98.
[2] hai li, qian shan, wu yong, & liu guangming. (2009). 3-(3-chloropropyl)-1,3,4,5-tetrahydro-7,8-dimethoxy -improvement of the synthesis process of 2h-3-benzazepine-2-one. west china journal of pharmaceutical sciences (05), 35-36.
[3] nanyun, huang meiyun, zhang lingling, & chen yingqi. (2013). synthesis of 3-amino-1,3,4,5-tetrahydro-2h-1-benzazepin-2-one . synthetic chemistry(03), 120-122.

 微信扫一扫打赏
微信扫一扫打赏

