background and overview[1]
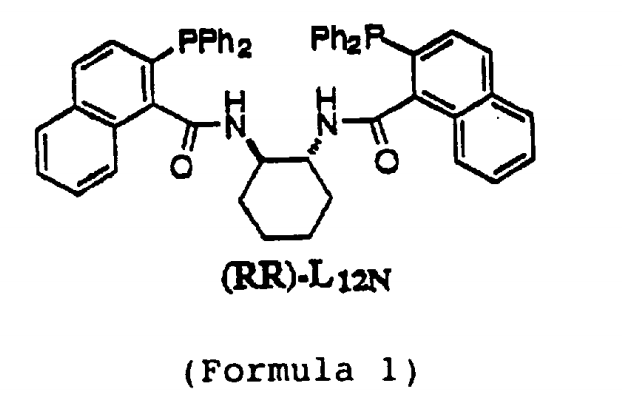
(1r,2r)-(+)-1,2-diaminocyclohexane-n,n’-bis(2-phenylphosphine-1-naphthoyl) is an organic phosphine ligand commonly used in transition metal catalyzed reactions.
this chiral coordination has proven to be very successful in the amination of butadiene monoepoxides, a type of epoxide and a potential prospect for asymmetric synthesis starting materials (or substrates).
preparation[1]
add 1.90g (5.33mmol) 2-diphenylphosphine-1-naphthoic acid and 42ml dichloromethane into a 100ml round-bottomed flask. after cooling to 0°c, add 1.78g (17.6mmol) triethylamine, and then add 1.58g (5.87mmol) diphenyl chlorophosphite dropwise within 2-3 minutes. after warming to room temperature over 5 hours, the mixture was transferred via cannula to 304 mg (2.66 mmol) (ir, 2r)-diaminocyclohexane and 30.5 mg (0.25 mmol) 4-dimethylaminopyridine in 11 ml dichloro methane solution and stir overnight. the reaction mixture was diluted with 50 ml of methylene chloride, washed with 1 x 50 ml of saturated aqueous sodium bicarbonate solution, dried over sodium sulfate and concentrated in vacuo. the crude product was purified by flash chromatography on silica gel (4.5 cm × 11 cm, 25% ethyl acetate in hexane) to give 1.07 g (51%) of a white solid, which crystallized from chloroform/hexane as a white powder (mp. 148- 150°c).
[α]d = +13.94° (c 1.19, ch2ci2). rf 0.64 (50% ethyl acetate in hexanes). ir (solution cdcl3): 3412, 3072, 3057, 2938, 2862, 1648, 1602, 1510, 1435, 1313, 1256, 1237, 1091, 1027 cm-1.
1hnmr (300 mhz, cdcl3) δ 7.8 (d, j=8.2 hz, 2h), 7.7 (d, j=9.1 hz, 2h) , 7.6 (d, j=8.9 hz, 2h), 7.2-7.4 (m, 22h),7.0 (m, 4h), 6.6 (d, j=5.5 hz, 2h), 3.8 (m, 2h), 2.3 ( m, 2h), 1.7 (m, 2h), 1.2-.13 (m, 4h).13c nmr (75 mhz, cdcl3) δ 169.2 (d , j=4.2 hz), 142.0 (d, j=34.2 hz), 136.85 (d, j=11.3 hz), 136.81 (d, j=11.3 hz), 133.5 (d, j=19.6 hz), 133.4 (d , j=19.3 hz), 133.3, 131.3 (d, j=18.1 hz), 129.9 (d, j=7.9 hz), 129.4, 129.2, 128.7, 128.7, 128.6, 128.5, 128.4 (d, j=6.7 hz) , 127.7, 127.2, 126.9, 125.6, 54.7, 31.5, 24.4.analysis: calcd for c, 78.97; h, 5.61; n, 3.54; p, 7.83. found: c, 78.76; h, 5.86; n, 3.38; p , 7.67.
references
[1] wipo patent application wo/1996/009306

 微信扫一扫打赏
微信扫一扫打赏

