background and overview[1]
2-cyano-4-nitro-6-bromoaniline is a very important disperse dye intermediate and an important raw material for the synthesis of disperse dyes such as disperse blue 183.
preparation[1]
(1) synthesis of 2-cyano-4-nitroaniline:
a1. put 100g o-chlorobenzonitrile into 333g sulfuric acid medium, then add mixed acid dropwise, and perform nitrification at 5-10°c to obtain nitrates; the mixed acid is hno3 and h2so4 is mixed with a mass percentage of 0.5:1;
a2. add water to hydrolyze the nitrate in step a1, and then filter it to obtain the nitrification material;
a3. dissolve the nitration material in step a2 in 400g chlorobenzene to obtain a nitric chlorine mixture;
a4. carry out an ammonolysis reaction between the nitric chlorine mixture and liquid ammonia in step a3. after the reaction reaches the end point, distill and recover the solvent at 80°c; the ammonolysis reaction temperature is 110°c, and the ammonolysis reaction pressure is 2.6mpa; reaction the end point is measured in a liquid chromatograph;
a5. filter the solvent in step a4 and wash it with water until neutral to obtain a wet product, namely 2-cyano-4-nitroaniline wet product;
the reaction equation in step (1) is:
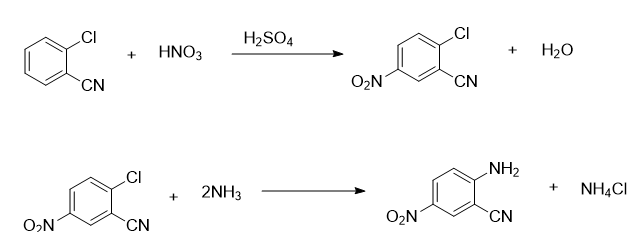
it can be seen that the sulfuric acid medium essentially acts as a solvent and provides acidic conditions for the reaction, and sulfuric acid does not participate in the reaction.
(2) synthesis of 2-cyano-4-nitro-6-bromoaniline:
b1. weigh 100g of 2-cyano-4-nitroaniline prepared in step a5 and put it into 20g of sulfuric acid medium, then dropwise add hydrogen bromide and 50g of hydrogen peroxide to react;
b2. after the reaction in step b1 reaches the endpoint through endpoint control, filter it to obtain the wet product of 2-cyano-4-nitro-6-bromoaniline.
the reaction equation in step (2) is:

similarly, the sulfuric acid medium essentially acts as a solvent, providing acidic conditions for the reaction to proceed, and sulfuric acid does not participate in the reaction.
references
[1] [chinese invention, chinese invention authorization] cn201410524535.9 a synthesis process of 2-cyano-4-nitro-6-bromoaniline

 微信扫一扫打赏
微信扫一扫打赏

