background and overview[1]
(s)-n-glycidyl phthalimide is also called s-glycidyl phthalimide amine. (s)-n-glycidyl phthalimide is an important intermediate for the preparation of rivaroxaban, new antibiotic linezolid, antifungal thioxazolidinones, and oxazolidinediones.
preparation[1]
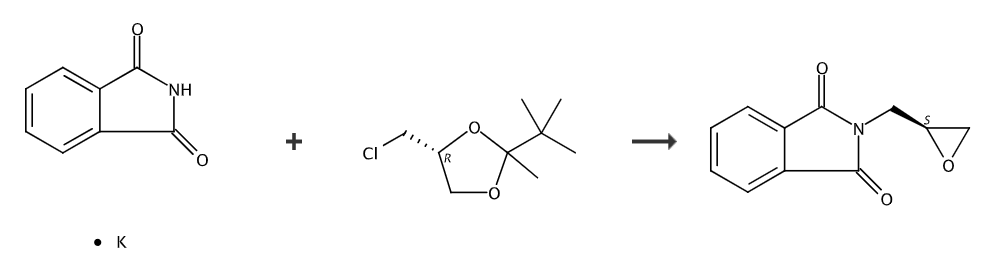
step 1, n-(s-2,2-dimethyl-1,3-dioxolane-4-)methylphthalamide preparation of amines
into a 1-liter four-necked flask connected to a stirrer, thermometer, and condenser tube, add 250 grams of n,n-dimethylformamide (dmf), 37.0 grams (0.20 mol) of ortho potassium phthalimide, 33.3 g (0.22 mol) r-2,2-dimethyl-4-chloromethyl-1,3-dioxolane, 0.5 g potassium iodide, heat and keep the internal temperature at 80 stir for 4 hours at -85°c. cool to 20°c, filter, wash the filter cake with 50 grams of dmf, and combine the filtrate. dmf was recovered by distillation under reduced pressure, and the residue was recrystallized with 80 g of methyl tert-butyl ether to obtain 49.7 g of n-(s-2,2-dimethyl-1,3-dioxolane-4-)methyl ortho phthalimide, yield 95.3%, hplc purity 99.7%.
step 2, preparation of s-glycidyl phthalimide
into a 500 ml four-neck bottle connected to a stirrer and thermometer, add 200 grams of 1,2 -dichloroethane, 26.5 g (0.10 mol) n-(s-2,2-dimethyl-1,3-dioxolane-4-)methylphthalimide, 25 g 40 % hydrobromic acid, stir and react for 4 hours at 10-15°c. separate the layers, extract the aqueous layer three times with 1,2-dichloroethane, 20 grams each time, combine the organic phases, and transfer the organic phases to a constant pressure low liquid funnel. in another flask, add 30 grams (0.15 mol) of 27% sodium methoxide methanol solution, and add the obtained organic phase dropwise while maintaining the internal temperature between 0-5°c. after the dripping is completed, stir and react at 10-15°c for 3 hours. add 200 grams of ice water, separate the layers, and extract the water layer twice with 1,2-dichloroethane, 20 grams each time. combine the organic phases, recover 1,2-dichloroethane by distillation, and use 50 grams of formazan as the residue. recrystallization of s-glycidyl tert-butyl ether gave 19.0 g of s-glycidyl phthalimide, with a yield of 93.6% and a hplc purity of 99.9%.
references
[1] [chinese invention] cn201710653271.0 a low-cost, high-purity synthesis method of s-glycidyl phthalimide

 微信扫一扫打赏
微信扫一扫打赏

