background and overview[1]
2-hydroxy-5-chlorobenzophenone can be used as a pharmaceutical synthesis intermediate. it can be prepared from 4-chlorophenyl benzoate as the reaction raw material, or by the reaction of substituted salicylic acid and arylboronic acid. .
preparation[1-2]
report 1,
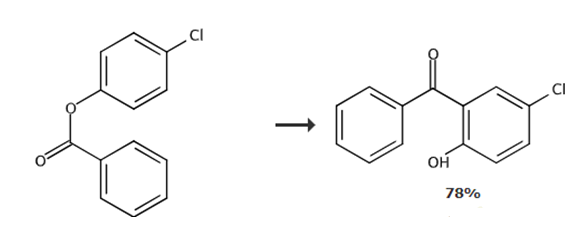
add 4-chlorophenyl benzoate (23.3 g, 100 mmol) and anhydrous aluminum chloride (16.7 g, 125 mmol, 1.25eq) into a 250 ml round-bottomed flask. the melted reaction mixture was slowly stirred at 160-180°c for 3 hours. the mixture was cooled to room temperature. quench the mixture with ice-cold water (170 ml) and 10% aqueous hydrochloric acid solution (85 ml), add dichloromethane (200 ml) to the mixture, and shake well until completely dissolved. transfer the solution to a separatory funnel, separate the aqueous layer from the organic layer, and extract twice more (2×200 ml). the combined organic layers were concentrated under reduced pressure to approximately 150 ml. transfer the mixture to a separatory funnel and extract the mixture with 2.5% sodium hydroxide solution (4×250 ml). acidify the combined alkaline aqueous layer with 10% sulfuric acid (ph≈1, approximately 300 ml), filter out the crude precipitate, and dry the filtrate in air. after purification with activated carbon, the crude crystals were crystallized from methanol to obtain 2-hydroxy-5-chlorobenzophenone.
report 2,
dissolve a mixture of substituted salicylaldehyde (0.2 mmol) and arylboronic acid (0.4 mmol) in dmf (2 ml). add [cp*rhcl2]2 (4.9 mg, 4 mol%) and cu(oac)2 (72.8 mg, 0.4), the reaction was carried out in a sealed tube at 80°c for 8 hours. the reaction mixture was diluted with ethyl acetate (20 ml) and washed with h2o (310 ml). the organic layer was washed with anhydrous na2so4dry. the crude product was loaded onto a silica gel column and flashed with 5-10% ethyl acetate in petroleum ether to give 2-hydroxy-5-chlorobenzophenone.
references
[1] series of high spin mononuclear iron(iii) complexes with schiff base ligands derived from 2-hydroxybenzophenones
[2] rh(iii)-catalyzed aldehyde c-h bond functionalization of salicylaldehydes with arylboronic acids

 微信扫一扫打赏
微信扫一扫打赏

