background and overview[1][2]
o-ethylphenylhydrazine hydrochloride is an intermediate in the synthesis of the anti-inflammatory and analgesic drug etodolac. in recent years, foreign research on this type of drug has progressed rapidly. since the mid-1980s, there have been mature patents and various aspects of research are quite sufficient. however, domestic reports are relatively rare. there are experimental synthesis of raw materials used for o-ethylphenylhydrazine. it is o-ethylaniline, which is produced by the reduction of o-nitroethylbenzene, a by-product of chloramphenicol produced by dongyao group.
first, o-ethylaniline reacts with sodium nitrite to form o-ethylaniline diazonium salt. the available reducing agents for reducing this diazonium salt to hydrazine include: sncl2·2h2o, so2. among them, the sncl2·2h2o reduction method is relatively mature and has a high yield, up to 70%. however, sncl2·2h2o is relatively expensive, the reduction time is long, and the low-temperature reaction must always be maintained. the high cost is not suitable for industrial production.
apply[3]
o-ethylphenylhydrazine hydrochloride is an intermediate for the synthesis of etodolac, an anti-inflammatory and analgesic drug. in addition, it can also be used as an intermediate for organic synthesis. examples of its application are as follows: preparation of 7-ethyl tryptol, 7- ethyl tryptol is a key intermediate for etodolac, the raw material of non-steroidal anti-inflammatory drugs. the preparation method includes: (1) hydrolyzing 2,3-dihydrofuran in a glycol ether solvent under acidic conditions to obtain 4-hydroxyl butyraldehyde; (2) 4-hydroxybutyraldehyde and o-ethylphenylhydrazine hydrochloride react in a glycol ether solvent in a temperature range of -20°c – the boiling point of the solvent for 1 to 24 hours, and then post-processed to obtain 7- ethyl tryptol. the preparation method of 7-ethyl tryptol of the present invention has good yield, convenient operation and high product purity, thereby further preparing etodolac and is suitable for industrial production.
preparation [1-2]
method 1: use o-ethylnitrobenzene, a by-product of the production of p-ethylnitrobenzene, as the raw material, triethylbenzylammonium chloride as the phase transfer catalyst, and use sodium sulfide to reduce it to o-ethylaniline (2 ). then 2 is diazotized, and o-ethyldiazobenzene is reduced with sodium sulfite to obtain o-ethylphenylhydrazine sulfonate, and hydrochloric acid is added to precipitate 1. based on o-ethylnitrobenzene, the total yield is 86.4%,
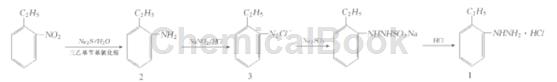
step 1: preparation of o-ethylaniline (2)
add water (300 ml), na2s (300 g, 3.85 mol), o-ethylnitrobenzene (302 g, 2 mol), and triethylbenzylammonium chloride (9g, 0.039 mol) in sequence. slowly raise the temperature to reflux, and use gc to track the reaction. the reaction is completed in about 8 hours. cool to about 50°c and stand to separate the supernatant liquid 2 (232 g, 96%), with a content of 98.2% (shimadzu gc-14 b gas chromatograph, column c pb1-m25-025, column temperature 160℃, the hydrogen flame detector temperature is 280℃, the injector temperature is 220℃, n2 is the carrier gas, the flow rate is 1 ml/min), and it can be directly used for the next step of synthesis without distillation.
step 2: synthesis of o-ethylphenylhydrazine hydrochloride (1)
in a 500 ml three-neck flask, add 31% industrial: hydrochloric acid (147 g, 1.25 mol), water (100 ml), and add 2 (60. 9g 0. 5 mol) dropwise at about 10°c. 30 minutes to complete. add a solution of sodium nitrite (34.5 g, 0.5 mol) and water (50 ml) dropwise at 0 to 5°c, use starch potassium iodide test paper to detect the end point of the reaction, stir for 10 minutes and set aside. in another 1000 ml four-necked flask, add sodium sulfite (157.5 g, 1.25 mol), water (300 ml) and 31% hydrochloric acid (50 ml). add the above solution at 85~90°c. complete the addition in 40 minutes and continue. reaction 2 h. add activated carbon (5 g), decolorize for 1 h, and filter. add hydrochloric acid (130ml) to the filtrate at 95°c and stir for 30 minutes. a large amount of sulfur dioxide gas is released at this time (absorbed with alkali solution). then cool to 0 ℃, pass
filter, wash with 10% hydrochloric acid (50 ml×3), and recrystallize with water to obtain white flaky crystals 1 (77.7 g, 89.5%), mp182~183℃ (literature [1] 181 ~ 183℃). ir and 1 hnmr are consistent with the structural characteristics, and the deviation between the experimental values and calculated values of elemental analysis (c8 h13 cln2) c, h, and n is less than 0.3%.
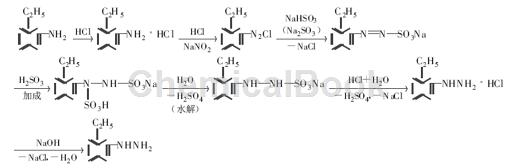
method 2: add 18 ml concentrated hydrochloric acid and 18 ml water to the three-necked flask (24 ml concentrated hydrochloric acid and 24 ml water are required when using na2so3 as the reducing agent), cool to below 0 ℃ in an ice-salt bath, and slowly drip add 10.9 g of o-ethylaniline, then add a solution of 6.2 g of sodium nitrite and 14.5 ml of water, use starch ki test paper to check the reaction end point, and use the β-naphthol penetration circle test to check the presence of diazonium salt. the reaction is complete. then add 37.4 g nahso3 and 7.2 g naoh and 80 ml water (when using na2so3 as reducing agent, add 45.3 g na2so3 and 130 ml water), heat to 80 ℃ to form a nahso3-na2so3 solution, keep the water bath temperature at 75~80 ℃, and dissolve the diazo in about 1 hour after adding the liquid, continue stirring for 2 h to complete the reaction.
add 47 ml of concentrated hydrochloric acid to the product, let it stand and raise the temperature to 85~90°c, then cool n for 5 minutes, and light pink crystals precipitate. after suction filtration, decolorization, hot filtration and recrystallization, white o-ethylbenzene is obtained hydrazine hydrochloride. add sodium hydroxide solution to this solid. the upper layer of the solution is an orange-yellow oil layer. after extraction with ether, light yellow flake crystals or orange-yellow oily liquid are obtained, which is o-ethylphenylhydrazine. the yield is 56.4% (using na2so3 as 52.5%).
main reference materials
[1] synthesis of o-ethylphenylhydrazine by sodium sulfite reduction method
[2] synthesis of o-ethylphenylhydrazine hydrochloride
[3] cn200510060465.7 preparation method of 7-ethyl tryptanol

 微信扫一扫打赏
微信扫一扫打赏

