background and overview[1]
2,3-diamino-6-fluorotoluene can be used as a pharmaceutical synthesis intermediate. if 2,3-diamino-6-fluorotoluene is inhaled, please move the patient to fresh air; if there is skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical treatment if you feel uncomfortable; if eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
preparation[1]
the preparation of 2,3-diamino-6-fluorotoluene is as follows:
step 1: synthesis of n-(3-fluoro-2-methylphenyl)acetamide
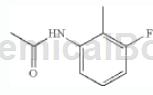
dissolve 1-amino-3-fluoro-2-methylbenzene (1 equiv) in dichloromethane and slowly add acetic anhydride (2.0 equiv). the solution was stirred at room temperature for 4 hours. the reaction mixture was then quenched with water and the aqueous layer was extracted with ethyl acetate. wash the organic layer with h2o, 10% hcl solution, h2o and brine. it was then dried over na2so4 and concentrated in vacuo to give n-(3-fluoro-2-methylphenyl)acetamide as a pink solid. lc/ms (m/z) 168.2 (mh+), rt 1.91 minutes.
step 2: synthesis of 1-amino-3-fluoro-2-methyl-4-nitrobenzene
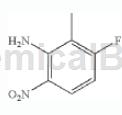
add the mixture of hno3/h2so4 (1:1, 60% hno3: concentrated h2so4) cooled to 0°c dropwise into n-(3-fluoro-2-methylphenyl)acetamide to form 0.16m solution. the solution was stirred at 0°c for 10 minutes and then at room temperature for 30 minutes. the solution was then diluted with water and made alkaline (ph=10) by adding 6nnaoh. the mixture was then extracted with ch2cl2 (3x), dried over na2so4, and concentrated in vacuo to give 1-amino-3-fluoro-2-methyl-6-nitrobenzene (the acetyl group was removed during basic workup). ). lc/ms (m/z) 171.1 (mh+), rt 1.87 minutes.
step 3: synthesis of 2,3-diamino-6-fluorotoluene
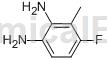
suspend 1-amino-3-fluoro-2-methyl-6-nitrobenzene (1.0 equiv) and 10% pd/c (0.1 equiv) in absolute ethanol at room temperature. the reaction flask was evacuated and then filled with h2. the resulting mixture was then stirred overnight under a hydrogen atmosphere. the resulting solution was filtered through celite and concentrated under vacuum to give 2,3-diamino-6-fluorotoluene. lc/ms (m/z) 141.1 (mh+), rt 0.43 minutes.
main reference materials
[1]us20060079564 indazole benzimidazole compounds

 微信扫一扫打赏
微信扫一扫打赏

