background and overview[1]
5-fluoro-2-methylphenylmagnesium bromide can be used as a pharmaceutical synthesis intermediate.
preparation[1]
generation of grignard reagent 5-fluoro-2-methylphenylmagnesium bromide in a single cstr: activate a slurry of magnesium (227g, 9.4mol) in methf (1l) to prepare 1.0mint1 grignard reagent (1207g) , occurs immediately after activating stirring. to compound 1 (23% (w/w)), methf was added to the cstr at a rate of 34.5 ml/min with a steady state τ of 0.5 h and a vessel temperature of 40 °c. the reaction solution exits via a dip tube and is used to isolate the solid magnesium in the cstr, and the solution is then collected in an accumulation vessel at a 1x rate (34.5 ml/min). the operation ran as a start/stop operation over 5 days, with a total processing time of 32 hours. pause overnight. the yield of 5-fluoro-2-methylphenylmagnesium bromide was 1.68 kg (69.6 mol). in total, there were 30 liters of 5-fluoro-2-methylphenylmagnesium bromide grignard reagent (97% conversion, 95% yield).
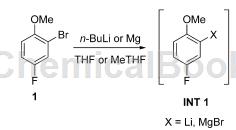
application
5-fluoro-2-methylphenylmagnesium bromide can undergo the following reactions:
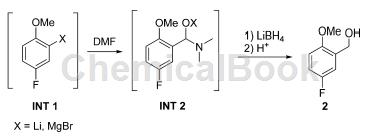
continuously pump 5-fluoro-2-methylphenylmagnesium bromide grignard reagent (1.02m) at 11.67ml/min simultaneously into 10% (w/w) dmf in thf, 11.67ml/ min. add 4mlibh4, continuously pump the solution in thf into cstr2 at 1.56ml/min, operate at 22°c for 0.5 hours, add 25% (w/w) methanol and h2so4, the concentration is 15.1ml/min. add toluene, combine several layers, wash with nahco3 aqueous solution, and concentrate to oil (3.93kg, yield 82.4%). cyclohexane (1400ml) was added, the mixture was filtered, the wet filter cake was washed with cyclohexane (1000ml), and the solid was dried at 22°c for 17 hours to obtain 555g of compound 2 (recovery rate 95.4%, total yield 50%) 78%) is a white powder with a purity of 99.9%.
main reference materials
[1] flowgrignardandlithiation: screening tools and development of continuous processes for a benzylalcoholstartingmaterial

 微信扫一扫打赏
微信扫一扫打赏

