background and overview[1]
1-(4-chlorophenyl)-1-cyclopentanecarboxylic acid is a formic acid derivative and can be used as a pharmaceutical synthesis intermediate. if 1-(4-chlorophenyl)-1-cyclopentanecarboxylic acid is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing and wash skin thoroughly with soap and water, if any if you feel unwell, seek medical attention.
preparation method[1]
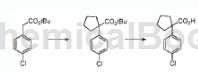
the preparation of 1-(4-chlorophenyl)-1-cyclopentanecarboxylic acid is as follows:
method 1: add benzyltriethylammonium chloride (0.025 equiv) and the appropriate dihalo compound (2.5 equiv) to substituted phenylacetonitrile. the mixture was heated at 70 °c and 50% sodium hydroxide (10 equiv.) was slowly added to the mixture. the reaction was stirred at 70°c for 12-24 hours to ensure complete formation of the cycloalkyl moiety, then heated at 130°c for 24-48 hours to ensure complete conversion from nitrile to carboxylic acid. the dark brown/black reaction mixture was diluted with water, extracted with ethyl acetate and then three times with dichloromethane to remove by-products. the alkaline aqueous solution was acidified with concentrated hydrochloric acid to a ph less than 1, and the precipitate that started to form at ph 4 was filtered and washed twice with 1m hydrochloric acid. the solid material was dissolved in methylene chloride, extracted twice with 1m hydrochloric acid and once with saturated aqueous sodium chloride solution. the organic solution was dried over sodium sulfate and evaporated to dryness to give cycloalkylcarboxylic acid 1-(4-chlorophenyl)-1-cyclopentanecarboxylic acid.
method 2: combine tert-butyl 1-(4-chlorophenyl)cyclopentanecarboxylate (545 mg, 1.94 mmol) with dcm (4 ml) and tfa (2 ml) and stir at room temperature. the reaction mixture was then evaporated onto silica and purified by flash chromatography to give the title compound 1-(4-chlorophenyl)-1-cyclopentanecarboxylic acid as a white solid (153 mg).
main reference materials
[1](wo2007056341)heterocyclicmodulatorsofatp-bindingcassettetransporters
[2](wo2014159224)histonedeacetylaseinhibitorsandcompositionsandmethodsofusethereof

 微信扫一扫打赏
微信扫一扫打赏

