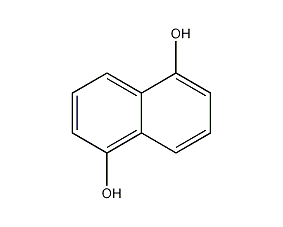
structural formula
| business number | 01u3 |
|---|---|
| molecular formula | c10h8o2 |
| molecular weight | 160.17 |
| label |
1,5-dihydroxynaphthalene, 1,5-naphthodiol, 1,5-dihydroxynaphthalene, naphthalene-1,5-diol, 1,5-naphthalenediol, c10h6(oh)2 |
numbering system
cas number:83-56-7
mdl number:mfcd00003980
einecs number:201-487-4
rtecs number:qj4740000
brn number:2044951
pubchem number:24893367
physical property data
1. characteristics: white needle-like crystals.
2. density (g/ml,25/4℃): unsure
3. relative vapor density (g/ml,air=1): unsure
4. melting point (ºc): 265 ℃
5. boiling point (ºc,normal pressure): unsure
6. boiling point (ºc,5.2kpa): unsure
7. refractive index: uncertain
8. flashpoint (ºc): 252℃
9. specific optical rotation (º): unsure.
10. autoignition point or ignition temperature (ºc): unsure
11. vapor pressure (kpa,25ºc): unsure
12. saturated vapor pressure (kpa,60ºc3. isotonic ratio (90.2k):341.1
4. surface tension (dyne/cm):64.4
5. polarizability(10-24cm3):18.97
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 2
3. number of hydrogen bond acceptors: 2
4. number of rotatable chemical bonds: 0
5. number of tautomers: 3
6. topological molecule polar surface area 40.5
7. number of heavy atoms: 12
8. surface charge: 0
9. complexity: 140
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
none yet
storage method
this product should be sealed in store in a cool and dry place.
synthesis method
by1,5- naphthalenedisulfonic acid is obtained by alkali fusion and acidification. heat the sodium hydroxide solution to290℃, add while stirring1,5-naphthalenedisulfonic acid, in280-300after adding the temperature between ℃, continue stirring30min . cool and acidify with hydrochloric acid to ph6-7. cool, filter and refine to obtain1,5-dihydroxynaphthalene.
purpose
for organic synthesis, dye intermediates, pharmaceutical intermediates, photographic industry.
rial; mso-bidi-font-family: arial; mso-ansi-language: en-us; mso-fareast-language: zh-cn; mso-bidi-language: ar-sa”>℃, add while stirring1,5-naphthalenedisulfonic acid, in280-300after adding between ℃, continue stirring30min. cool and acidify with hydrochloric acid to ph6-7. cool, filter and refine to obtain1,5-dihydroxynaphthalene.
purpose
for organic synthesis, dye intermediates, pharmaceutical intermediates, photographic industry.

 微信扫一扫打赏
微信扫一扫打赏

