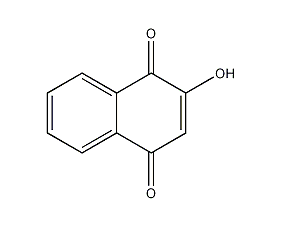
structural formula
| business number | 01u8 |
|---|---|
| molecular formula | c10h6o3 |
| molecular weight | 174.15 |
| label |
2-hydroxy-p-naphthoquinone, 2-hydroxy-p-naphthoquinone, lawsone, natural orange 6 |
numbering system
cas number:83-72-7
mdl number:mfcd00001678
einecs number:201-496-3
rtecs number:ql8200000
brn number:1565260
pubchem number:24895653
physical property data
1. characteristics: bright yellow prismatic crystals.
2. density (g/ml,25/4℃): undetermined
3. relative vapor density (g/ml,air=1): undetermined
4. melting point (ºc): 195-196℃(decomposition)
5. boiling point (ºc,normal pressure): undetermined
6. boiling point (ºc,5.2kpa): undetermined
7. refractive index: undetermined
8. flashpoint (ºc): undetermined
9. specific optical rotation (º): undetermined
10. autoignition point or ignition temperature (ºc): undetermined
11. vapor pressure (kpa,25ºc): undetermined
ecological data
none yet
molecular structure data
1. molar refractive index: 44.49
2. molar volume (m3/mol):117.5
3. isotonic specific volume (90.2k):343.8
4. surface tension (dyne/cm):73.2
5. polarizability(10-24cm3):17.63
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): 0.9
2. number of hydrogen bond donors: 1
3. number of hydrogen bond acceptors: 3
4. number of rotatable chemical bonds: 0
5. number of tautomers: 3
6. topological molecule polar surface area 54.4
7. number of heavy atoms: 13
8. surface charge: 0
9. complexity: 291
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
none yet
storage method
this product should be sealed and stored in a cool, dry place.
synthesis method
will1,2-naphthoquinone -4-ammonium sulfonate and sulfur react in methanol to obtain methoxynaphthalene quinone is then hydrolyzed with sodium hydroxide to obtain2-hydroxy-1,4-naphthoquinone. yield58-65%.
purpose
hair dye, fungicide .
number of stereocenters: 0
12. uncertain number of stereocenters of atoms: 0
13. determined number of stereocenters of chemical bonds: 0
14. uncertain number of stereocenters of chemical bonds: 0
15. number of covalent bond units: 1
properties and stability
none yet
storage method
this product should be sealed and stored in a cool, dry place.
synthesis method
will1,2-naphthoquinone -4-ammonium sulfonate and sulfur react in methanol to obtain methoxynaphthalene quinone is then hydrolyzed with sodium hydroxide to obtain2-hydroxy-1,4-naphthoquinone. yield58-65%.
purpose
hair dye, fungicide .

 微信扫一扫打赏
微信扫一扫打赏

