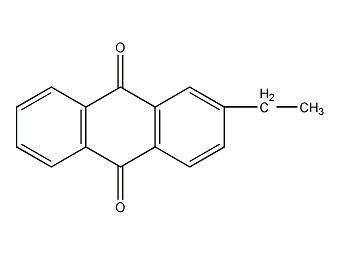
structural formula
| business number | 01um |
|---|---|
| molecular formula | c16h12o2 |
| molecular weight | 236.27 |
| label |
c6h4(co)2c6h3c2h5 |
numbering system
cas number:84-51-5
mdl number:mfcd00001237
einecs number:201-535-4
rtecs number:cb0525000
brn number:1969873
pubchem number:24894394
physical property data
1. character:light yellow solid
2. density (g/ml ,25/4℃): undetermined
3. relative vapor density (g/ ml,air=1 ): undetermined
4. melting point (ºc):107-111 ℃.
5. boiling point (ºc,normal pressure): undetermined
6. boiling point (ºc,5.2 kpa): undetermined
7. refractive index: undetermined
8. flash point (ºc): undetermined
9. specific optical rotation (º ): undetermined
10. autoignition point or ignition temperature (ºc): undetermined
11. vapor pressure (kpa, 25ºc): undetermined
1. moore refraction rate: 68.21
2. molar volume (m3/mol):191.8
3. isotonic specific volume (90.2k):513.1
4. surface tension (dyne/cm):51.1
5. polarizability(10-24cm3):27.04
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 0
3. number of hydrogen bond acceptors: 2
4. number of rotatable chemical bonds: 1
5. number of tautomers: 21
6. topological molecule polar surface area 34.1
7. number of heavy atoms: 18
8. surface charge: 0
9. complexity: 360
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
none yet
storage method
this product should be sealed in store in a cool and dry place.
synthesis method
none yet
purpose
generated by hydrogenation in solvent2-ethyl anthrahydroquinone, regenerated upon reoxidation2-ethyl anthraquinone and hydrogen peroxide, this reaction is used industrially to produce hydrogen peroxide. from phthalic anhydride and ethylbenzene through font-family–gram reaction, can also be produced by1,4-naphthoquinone and2-ethyl-1,3 –butadiene via diels–produced by alder diene synthesis and dehydrogenation reaction. it is used to prepare hydrogen peroxide and as a dye intermediate. it is also used as a sensitizer for photosensitive resins, photocurable resin catalysts, photodegradable films, coatings and photosensitive polymerization initiators
��: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine chemical bonds number of stereocenters: 0
14. number of stereocenters of uncertain chemical bonds: 0
15. number of covalent bond units: 1
properties and stability
none yet
storage method
this product should be sealed in store in a cool and dry place.
synthesis method
none yet
purpose
generated by hydrogenation in solvent2-ethyl anthrahydroquinone, regenerated upon reoxidation2-ethyl anthraquinone and hydrogen peroxide, this reaction is used industrially to produce hydrogen peroxide. from phthalic anhydride and ethylbenzene through font-family–gram reaction, can also be produced by1,4-naphthoquinone and2-ethyl-1,3 –butadiene via diels–produced by alder diene synthesis and dehydrogenation reaction. it is used to prepare hydrogen peroxide and as a dye intermediate. it is also used as a sensitizer for photosensitive resins, photocurable resin catalysts, photodegradable films, coatings and photosensitive polymerization initiators

 微信扫一扫打赏
微信扫一扫打赏

