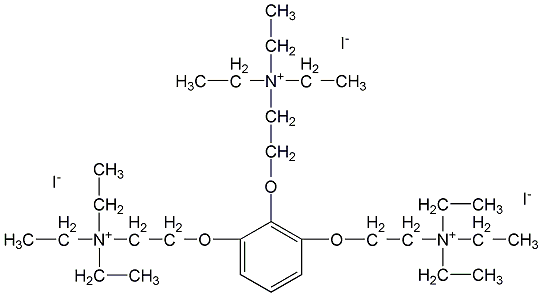
structural formula
| business number | 01dp |
|---|---|
| molecular formula | c30h60i3n3o3 |
| molecular weight | 891.54 |
| label |
triiodoquaternary ammonium phenol |
numbering system
cas number:65-29-2
mdl number:mfcd00011832
einecs number:200-605-1
rtecs number:bs1100000
brn number:none
pubchem number:24277837
physical property data
1. character:white or milky white crystalline powder, odorless, slightly bitter, hygroscopic
2. density (g/ml,25/4℃): unsure
3. relative vapor density (g/ml,air=1): unsure
4. melting point (ºc):235 (dec.)(lit.)
5. boiling point (ºc,normal pressure): uncertain
6. boiling point (ºc, 5.2kpa): unsure
7. refractive index:not sure
8. flash point (ºc): unsure
9. specific optical rotation (º): unsure
10. autoignition point or ignition temperature (ºc): unsure
11. vapor pressure (kpa,25ºc): unsure
12. 17. explosion limit (%, v/v): unsure
18. lower explosion limit (%,v/v): unsure
19. solubility:easily soluble in water(1:6), slightly soluble in ethanol(1:500), acetone, benzene and chloroform, insoluble in ether
toxicological data
none yet
ecological data
none yet
molecular structure data
none yet
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 0
3. number of hydrogen bond acceptors: 6
4. number of rotatable chemical bonds: 21
5. number of tautomers: none
6. topological molecule polar surface area 27.7
7. number of heavy atoms: 39
8. surface charge: 0
9. complexity: 489
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 4
properties and stability
none yet
storage method
this product should be sealed in4℃save in a dry place and away from light.
synthesis method
pyrogallic acid forms a sodium salt under the action of sodium acetate , and then reacted with diethylaminoethyl chloride and ethyl iodide in sequence.
purpose
for biochemical research. medicine.
eft> this product should be sealed in 4℃keep dry and away from light.
synthesis method
pyrogallic acid forms a sodium salt under the action of sodium acetate , and then reacted with diethylaminoethyl chloride and ethyl iodide in sequence.
purpose
for biochemical research. medicine.

 微信扫一扫打赏
微信扫一扫打赏

