background and overview[1]
the direct coupling of phenols such as 2,2′-biphenol to the corresponding biphenol derivatives of industrial importance has always been a challenge because these reactions typically have neither regioselectivity nor chemical options sex. the term phenols is used in this application as a class concept and therefore also includes substituted phenols. 2,2”-biphenol can be used in the synthesis of pharmaceutical intermediates.
structure
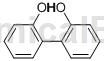
apply[2-3]
2,2”-biphenol can be used to synthesize pharmaceutical intermediates:
1) preparation of tris(2,2”-biphenol)bisphosphite. this method uses trialkyl phosphite and 2,2′-biphenol in a molar ratio of 1:1-10:1. as the raw material, aryl-substituted phosphorus chloride or alkyl-substituted phosphorus chloride is used as the catalyst, and bisphosphite tris(2,2”-biphenol) ester is synthesized under solvent-free microwave radiation conditions. the microwave radiation power is 100-1000w. microwave reaction time is 5-30 minutes. the process of the invention has the characteristics of high bisphosphite yield, less by-products, short reaction time, solvent-free operation, environmental friendliness, no pollution, and simple post-processing.
2) prepare bisphosphite. in this method, reagent amounts of phosphorus trichloride, 2,2′-biphenol and 3,3′,5,5′-tetratert-butyl-2,2′- using biphenol as the raw material, dissolve phosphorus trichloride in an organic solvent to form a solution, and then add the solution containing 2,2′-biphenol and the catalyst under stirring. after the addition of the phosphorus trichloride solution is completed, the reaction solution continues to react. several hours; add a solution of 3,3′,5,5′-tetratert-butyl-2,2′-biphenol and organic base to the above reaction solution. after the addition is completed, the reaction solution continues to stir for several hours, and then raise the temperature to 80-120°c and reflux for several hours; after the reaction is completed, the reaction solution is cooled to room temperature, filtered, and washed. the filter cake is combined, and the filtrate is evaporated and concentrated to obtain a solid. after washing, the product is obtained. the process of the invention has the characteristics of high yield of bisphosphite, low consumption of phosphorus trichloride, less by-products, short reaction time, and simple post-processing.
preparation[1]
the method of 2,2′-biphenol includes the following steps:
a) add the first phenol to the reaction mixture,
b) add the second phenol to the reaction mixture,
c) add selenium dioxide to the reaction mixture,
d) add an acid with a pks value in the range of 0.0 to 5.0 into the reaction mixture,
e) heating the reaction mixture converts the first phenol and the second phenol into 2,2′-biphenol.
main reference materials
[1] cn201510386228.3 method for preparing 2,2’-biphenol using selenium dioxide
[2] cn201310470257.9 a microwave synthesis method of bisphosphite tris(2,2’-biphenol) ester
[3] cn201811350926.8 a synthesis method of bisphosphite

 微信扫一扫打赏
微信扫一扫打赏

