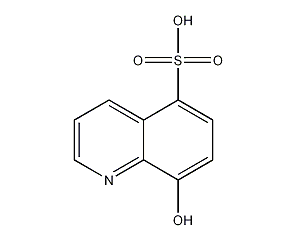
structural formula
| business number | 01v8 |
|---|---|
| molecular formula | c9h7no4s |
| molecular weight | 225.22 |
| label |
8-hydroxy-5-quinolinesulfonic acid, 5-sulfonic acid-8-hydroxyquinoline, 5-sulfo-8-hydroxyquinoline |
numbering system
cas number:84-88-8
mdl number:mfcd00006795
einecs number:201-570-5
rtecs number:vc2570000
brn number:none
pubchem number:24895427
physical property data
1. character:light yellow needle-like crystals or crystalline powder
2. density (g/ml ,25/4℃): undetermined
3. relative vapor density (g /ml,air= 1): undetermined
4. melting point (ºc):213℃(decomposed)
5. boiling point (ºc,normal pressure): undetermined
6. boiling point (ºc,5.2 kpa): undetermined
7. refractive index: undetermined
8. flash point (ºc): undetermined
9. specific optical rotation (º ): undetermined
10. autoignition point or ignition temperature (ºc): undetermined
11. vapor pressure (kpa, 25ºc): undetermined
salmonella: 50 ug/l
ecological data
none yet
molecular structure data
1. molar refractive index:53.98
2. molar volume (m3/mol):137.9
3. isotonic specific volume (90.2k):415.9
4. surface tension (dyne/cm):82.5
5. polarizability(10-24cm3):21.40
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 2
3. number of hydrogen bond acceptors: 5
4. number of rotatable chemical bonds: 1
5. number of tautomers: 2
6. topological molecule polar surface area 95.9
7. number of heavy atoms: 15
8. surface charge: 0
9. complexity: 324
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
none yet
storage method
this product should be sealed in store in a cool and dry place.
synthesis method
by8-original sulfonation of hydroxyquinoline. cool the oleum with stirring to 10℃the following, gradually added8-hydroxyquinoline, keep the temperature at15℃below. after adding, raise the temperature to 30℃the following stirring reaction5h. leave overnight. the next day, put the reaction solution into 8-10double the amount of water (press8-hydroxyquinoline meter), control temperature60℃, precipitate the crystals, and then leave it overnight. filter, wash and dry.
purpose
used as organic synthesis intermediates and analytical reagents.
s: list 18.0pt; mso-pagination: wi-orphan” align=left>
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 2
3. number of hydrogen bond acceptors: 5
4. number of rotatable chemical bonds: 1
5. number of tautomers: 2
6. topological molecule polar surface area 95.9
7. number of heavy atoms: 15
8. surface charge: 0
9. complexity: 324
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
none yet
storage method
this product should be sealed in store in a cool and dry place.
synthesis method
by8-original sulfonation of hydroxyquinoline. cool the oleum with stirring to 10℃the following, gradually added8-hydroxyquinoline, keep the temperature at15℃below. after adding, raise the temperature to 30℃the following stirring reaction5h. leave overnight. the next day, put the reaction solution into 8-10double the amount of water (press8-hydroxyquinoline meter), control temperature60℃, precipitate the crystals, and then leave it overnight. filter, wash and dry.
purpose
used as organic synthesis intermediates and analytical reagents.
mily: 宋体”>
purpose
used as organic synthesis intermediates and analytical reagents.

 微信扫一扫打赏
微信扫一扫打赏

