overview[1][2]
3-hydroxyanthranilic hydrochloride is an amino compound and can be used as a pharmaceutical synthesis intermediate.
structure
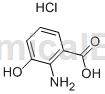
preparation[1-2]
method 1: 3-hydroxyanthranilic hydrochloride is prepared as follows: add 6.0g 85.3% h3po4 to a 100ml three-neck flask equipped with a mechanical stirrer, nitrogen outlet and oil bubbler. cool to 0°c in an ice bath. to this solution was added 9.72g p2o5 (88% p2o5 content). heat the viscous mass to 150°c and stir for 6 hours; then add pre-dried 1.7g haa (2.1mmol) and 1.7g 2-amino-3-hydroxybenzoic acid (2.1mmol). the temperature was raised to 185°c and the reaction was allowed to proceed under positive nitrogen pressure for an additional 18-32 hours. the resulting reaction mixture was precipitated in water, filtered and washed with water in a soxhlet extractor for 24 hours. the solid was placed in a vacuum oven and dried at 100 °c for 24 h, acidified with hydrochloric acid, and the product was characterized by ir, tga, and dsc, and the intrinsic viscosity of the material was measured.
method 2: use penicillium catalysis to prepare 3-hydroxyanthranilic hydrochloride. the specific steps are as follows: 15000l fermentation tank, that is, prepare a microbial fermentation liquid fermentation tank, and add the substrate 2-aminobenzoate to the fermentation liquid. phenol, the concentration is 90-95g/l, add the following ingredients, the concentration is: linear 10-carbon alcohol polyoxyethylene ether is 10-12g/l, xylose 20-24g/l, sucrose 25-28g/l l. glutinous rice flour 18-22g/l, autoclave at 121°c for 30 minutes; when cooled to 33-35°c, stir and emulsify, and perform biocatalytic reaction. the reaction time is 49-54 hours; when the reaction starts, add the concentration immediately it is 5% nahco3 aqueous solution, and at the same time, add 1% hydrochloric acid aqueous solution, adjust the flow acceleration rate of nahco3 and hydrochloric acid aqueous solution, and maintain ph 6.4-6.6 , the added materials have been sterilized; the ventilation ratio is 0.6-0.7v/v, that is, the ventilation volume per minute is 0.6-0.7 times the volume of the reaction solution. after the reaction is completed, break the emulsion and extract with a countercurrent extraction machine. it is ethyl acetate, the ethyl acetate in the organic phase is evaporated, and the ethyl acetate is evaporated to obtain the product 2-amino-3-hydroxy-benzoformic acid. the reaction conversion rate is 98.1-99.6%, and the product yield is 95.9-97.1%.
main reference materials
[1](us5340913)synthesisofaromaticheterocyclicpolymersfromabiosyntheticallypreparedprecursor
[2] (cn106399408) method for preparing amino-hydroxyl benzoic acid by adopting microbes

 微信扫一扫打赏
微信扫一扫打赏

