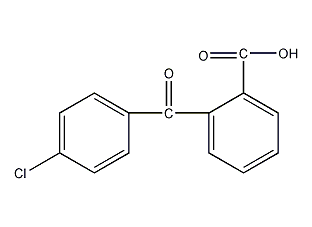
structural formula
| business number | 01w0 |
|---|---|
| molecular formula | c14h9clo3 |
| molecular weight | 260.67 |
| label |
2-(4-chlorobenzoyl)benzoic acid, o-(p-chlorobenzoyl)benzoic acid, cbb acid, 2-(4-chlorobenzoyl)benzoic acid, clc6h4coc6h4co2h |
numbering system
cas number:85-56-3
mdl number:mfcd00002474
einecs number:201-615-9
rtecs number:none
brn number:649894
pubchem number:24852765
physical property data
1. properties: crystal
2. density (g/ml, 25/4℃): undetermined
3. relative vapor density (g/ml, air = 1): undetermined
4. melting point (ºc): 150-151
5. boiling point (ºc, normal pressure): undetermined
6. boiling point (ºc, 5.2kpa): not determined
7. refractive index: not determined
8. flash point (ºc): not determined
9. specific rotation (º): undetermined
10. autoignition point or ignition temperature (ºc): undetermined
11. vapor pressure (kpa, 25ºc): undetermined
12. saturated vapor pressure (kpa, 60ºc): undetermined
13. heat of combustion (kj/mol): undetermined
14. critical temperature ( ºc): undetermined
15. critical pressure (kpa): undetermined
16. log value of oil-water (octanol/water) partition coefficient: undetermined
17. explosion upper limit (%, v/v): undetermined
18. explosion lower limit (%, v/v): undetermined
19. solubility: soluble in benzene, ether and ethanol.
toxicological data
none yet
ecological data
none yet
molecular structure data
1. molar refractive index: 67.87
2. molar volume (cm3/mol): 192.0
3. isotonic specific volume (90.2k): 524.9
4. surface tension (dyne/cm): 55.8
5. polarizability (10-24cm3): 26.90
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 1
3. number of hydrogen bond acceptors: 3
4. number of rotatable chemical bonds: 3
5. number of tautomers: none
6. topological molecule polar surface area 54.4
7. number of heavy atoms: 18
8. surface charge: 0
9. complexity: 321
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. uncertaintynumber of bonded stereocenters: 0
15. number of covalent bond units: 1
properties and stability
none yet
storage method
this product should be sealed and stored in a cool, dry place.
synthesis method
obtained from phthalic anhydride through freund-g reaction: add chlorobenzene and anhydrous aluminum trichloride to the reaction pot, and slowly add phthalic anhydride. after the addition is completed, keep it at 75-80°c for 2.5 hours, cool it, put the reaction solution into hydrochloric acid ice water to decompose, and let it stand for layering. the chlorobenzene layer was extracted three times with 5% sodium hydroxide solution, the extracts were combined, adjusted to ph 2-3 with hydrochloric acid, filtered, and dried to obtain cbb.
purpose
intermediates for medicines and dyes, used in the production of chlorthalidone, 2-chloroanthraquinone, etc.

 微信扫一扫打赏
微信扫一扫打赏

