background and overview[1]
bistriphenylphosphine nickel dicarbonyl can be used as pharmaceutical synthesis intermediates and organic intermediates, mainly used in laboratory research and development processes and chemical and pharmaceutical synthesis processes.
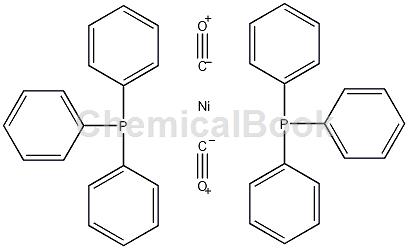
preparation[1]
in a drying box, dissolve l-leucine nca (9.2mg, 0.058mmol) in thf (0.5ml) and add evenly stirred pph3 (31mg, 0.12mmol) and (cod)2ni (16mg, 0.058) in the mixture. mmol) in thf (1.5 ml). the red/brown solution was stirred for 24 hours, then the solvent was removed in vacuo to give a dark red oily solid. the product was extracted with cold hexane (0°c, 3×2). a red/brown hexane solution and a light orange solid were obtained. evaporation of the hexane solution gave a red oil containing (pph3)2ni(co)2[ir(thf): 2000, 1939cm-1(nco).
the solid was then dried to give an orange powder, which could be purified by precipitation from thf/hexane to give bistriphenylphosphine nickel dicarbonyl (5 mg, 66% yield). the product was dissolved in thf (5 ml) in a round bottom schlenk flask in a dry oven. place the flask under a n2 atmosphere on the schlenk line and add hcl (90 ml of a 1.0 met2o solution). the yellow solution turns orange and then becomes hazy as it slowly turns green. after 2 hours, the solvent was removed in vacuo to obtain bistriphenylphosphine nickel dicarbonyl.
apply[2]
bistriphenylphosphine nickel dicarbonyl can be used as pharmaceutical synthesis intermediate: 3, 3, 4, 4, 5, 5, 6, 6, 6-nonafluorohex-1-ene (20.5 g, 0.0833 mol) , bistriphenylphosphine nickel dicarbonyl (0.53 g, 0.0008 mol), and perfluoroethyl iodide (153.6 g, 0.625 mol) were added to a 210 ml hastelloytm shaking tube and heated at 100°c for 8 hours under autogenous pressure. . analysis of the product by gc-ms showed the presence of c4f9chich2c2f5 (64.3 gc area %) and the diadduct (3.3 gc area %); 3,3,4,4,5,5,6,6,6-nonafluorohexane-1 the conversion rate of -ene is 80.1%.
main reference materials
[1]us6632922methods and compositions for controlled polypeptide synthesis
[2]wo2008097638laser-assistedetchingusinggascompositionscomprisingunsaturatedfluorocarbons

 微信扫一扫打赏
微信扫一扫打赏

