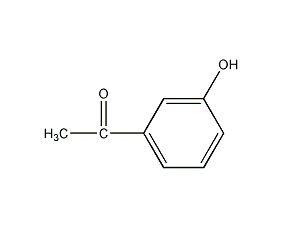
structural formula
| business number | 03dy |
|---|---|
| molecular formula | c8h8o2 |
| molecular weight | 136.15 |
| label |
m-hydroxyacetophenone, m-hydroxyacetophenone, m-acetylphenol, 3-hydroxyacetophenone, 3-acetylphenol, m-acetylphenol, ethanone, 1-(3-hydroxyphenyl)-, m-hydroxyacetophenonel, 3-acetylphenol, m-acetylphenol, aromatic compounds |
numbering system
cas number:121-71-1
mdl number:mfcd00002298
einecs number:204-494-0
rtecs number:am8574000
brn number:2040676
pubchem number:24859714
physical property data
1. characteristics: acicular crystal
2. relative density (109℃): 1.099
3. refractive index ( n109d): 1.5348
4. melting point (℃): 95-97
5. boiling point (ºc): 296
6. boiling point (ºc, 0.67kpa): 153
toxicological data
ecological data
none
molecular structure data
5. molecular property data:
1. molar refractive index: 38.16
2. molar volume (cm3/mol): 119.3
3. isotonic specific volume (90.2k): 307.4
4. surface tension (dyne/cm): 43.9
5. dielectric constant:
6. dipole moment (10-24cm3):
7. polarizability: 15.12
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 1
3. number of hydrogen bond acceptors: 2
4. number of rotatable chemical bonds: 1
5. number of tautomers: 6
6. topological molecule polar surface area 37.3
7. number of heavy atoms: 10
8. surface charge: 0
9. complexity: 131
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
1. exist inin the flowing smoke.
storage method
none
synthesis method
2. brief description of production method
obtained from the reaction of m-aminoacetophenone and sodium nitrite. add water and concentrated sulfuric acid into the reaction pot, add m-aminoacetophenone while stirring, and then add sodium nitrite aqueous solution dropwise while cooling, keeping the temperature at 8-10°c. after adding, slowly raise the temperature to above 90°c, and continue stirring. reaction 1h. the solid is precipitated by cooling, and is filtered. the obtained crude product is recrystallized with hot water to obtain m-hydroxyacetophenone. the yield is 65%.
purpose
3. use
it is used in organic synthesis and is an intermediate of the drug phenylephrine.

 微信扫一扫打赏
微信扫一扫打赏

