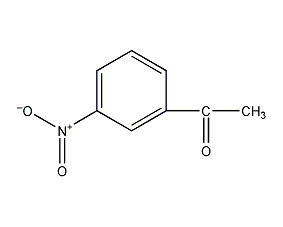
structural formula
| business number | 03e7 |
|---|---|
| molecular formula | c8h7no3 |
| molecular weight | 165.15 |
| label |
m-nitroacetophenone, 3-nitroacetophenone, 1-(3-nitrophenyl)ethanone, m-nitroacetophenone, 3-nitroacetophenone, 3′-nitroacetophenone, 98+%, m-nitroacetophenone, 3′-nitroacetophenone, 3-nitroacetophenone, akos bbs-00003220, akos 233-65, 1-(3-nitrophenyl)ethanone, labotest-bb lt01895210, aurora 14018, fragrance |
numbering system
cas number:121-89-1
mdl number:mfcd00007259
einecs number:204-504-3
rtecs number:am9625000
brn number:743002
pubchem id:none
physical property data
1. characteristics: yellow needle-like crystals.
2. melting point (℃):81℃
3. boiling point (ºc,2.0kpa):202℃, 167℃(239kpa).
4. solubility:easily soluble in hot water and ether, slightly soluble in ethanol. can evaporate with water vapor.
toxicological data
1, acute toxicity: rat oral administrationld50:3250mg/kg
mouse transperitoneal cavity ld50: 200mg/kg
rabbit skinld50:3ml/kg
2salmonella mutation test system: 1mg/plate
ecological data
none
molecular structure data
5. molecular property data:
1. molar refractive index:42.82
2. molar volume(m3/mol):132.8
3. isotonic specific volume(90.2k):347.9
4. surface tension(dyne/cm)47.1
5. dielectric constant:
6. dipole moment(10-24cm3) :
7. polarizability:16.97
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 0
3. number of hydrogen bond acceptors: 3
4. number of rotatable chemical bonds: 1
5. number of tautomers: 2
6. topological molecule polar surface area 62.9
7. number of heavy atoms: 12
8. surface charge: 0
9. complexity: 197
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
none
storage method
none
synthesis method
1. introduction to production methods
obtained from the nitration of acetophenone. additionally, with 2-nitroacetophenone the preparation method is similar, and this product can also be obtained by oxidation of m-nitroethylbenzene.
purpose
2. purpose
used as an intermediate in organic synthesis.
-size: 10pt; color: #333333; font-family: 宋体; mso-ascii-font-family: ‘times new roman’; mso-hansi-font-family: ‘times new roman'”>from acetophenone obtained by nitrification. in addition, with 2-nitro the preparation method of acetophenone is similar, and it can also be obtained by oxidizing m-nitroethylbenzene.
purpose
2. purpose
used as an intermediate in organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

