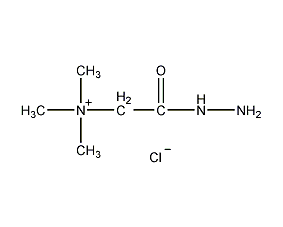
structural formula
| business number | 03gr |
|---|---|
| molecular formula | c5h14cln3o |
| molecular weight | 167.64 |
| label |
acetyl hydrazine trimethylammonium chloride, betaine chloride hydrazide, 2-hydrazino-n,n,n-trimethyl-2-oxoethylammonium chloride, (carboxymethyl)trimethylammonium chloride hydrazide, (hydrazinocarbonylmethyl)trimethylammonium chloride, aliphatic compounds |
numbering system
cas number:123-46-6
mdl number:mfcd00012009
einecs number:204-629-3
rtecs number:cp3150000
brn number:3727163
pubchem number:24895356
physical property data
1. properties: white needle-like crystals. strong hygroscopicity
2. melting point: 188-192℃ (decomposition)
3. solubility: easily soluble in water, soluble in 150 parts of absolute ethanol, soluble in ice acetic acid, glycerin and ethylene glycol
toxicological data
1. acute toxicity: rat oral ld: >500mg/kg
ecological data
none yet
molecular structure data
none yet
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 2
3. number of hydrogen bond acceptors: 3
4. number of rotatable chemical bonds: 2
5. number of tautomers: 2
6. topological molecule polar surface area 55.1
7. number of heavy atoms: 10
8. surface charge: 0
9. complexity: 105
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 2
properties and stability
1. properties: white needle-like crystals. highly hygroscopic.
storage method
none yet
synthesis method
1. preparation method: mix ethyl chloroacetate and absolute ethanol, cool to 0°c, add trimethylamine pre-cooled to -5°c, control the exothermic reaction, and raise the reaction temperature to 60°c, after stopping the heat generation, place it at room temperature for 24 hours, add dropwise 100% hydrazine hydrate, stir for 45 minutes, cool, filter out the crystals, and dry to obtain giralde’s reagent t, with a yield of 83.5%-89.5%.
2. add 98.5 grams (84.5 liters, 0.8 mol) ethyl chloroacetate and 200 ml of absolute ethanol. cool to 0°c, stop stirring, add 49 grams (74 ml, 0.83 mol) of trimethylamine pre-cooled to -5°c at one time, and use cooling to fully control the release.carry out the reaction so that the temperature of the reactants rises to 60°c within about 10 working hours. after no longer exothermic, let the reactants stand at room temperature for 20 to 24 hours. remove the condenser, replace the thermometer with a dropping funnel, add 40 grams (0.8 mol) of 100% hydrazine hydrate dropwise within 10-15 minutes under stirring, and continue stirring for 45 minutes. after the solution is slightly cooled, if the product does not crystallize automatically, rub the bottle wall with a glass rod to stimulate crystallization. the product will appear as fine colorless needle-shaped crystals. cool in an ice bath, quickly filter out the easily deliquescent salt, wash with 1}} liters of absolute ethanol, press dry, and dry in a vacuum dryer filled with concentrated sulfuric acid. the product weighs 100 to 108 grams. after evaporating 200-300 ml of solvent from the mother liquor, a second batch of products was obtained, with a total weight of 112-120 grams (83.5-89.5%). the product should be stored in a dry, airtight container. after the product has been placed for a period of time,
it is best to recrystallize it with absolute ethanol before use.
purpose
1. used to separate estrone and 17-steroid compounds.
2.separate ketones from mixtures containing non-ketone substances. also used in some dehydration reactions.

 微信扫一扫打赏
微信扫一扫打赏

