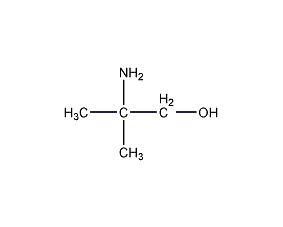
structural formula
| business number | 03jf |
|---|---|
| molecular formula | c4h11no |
| molecular weight | 89.14 |
| label |
2-aminoisobutanol, butylaminol, isobutanolamine, β-aminoisobutyl aroma, β-aminoisobutyl alcohol, amp, β-amino-iso-butyl alcohol, 2,2-diethylethanolamine, 2-hydroxymethyl-2-propylamine, 2-methyl-2-amino-1-propanol, aliphatic compounds |
numbering system
cas number:124-68-5
mdl number:mfcd00008051
einecs number:204-709-8
rtecs number:ua5950000
brn number:505979
pubchem number:24862008
physical property data
1. properties: colorless and transparent liquid, or white petroleum jelly-like substance with a special smell.
2. density (g/ml, 25/4℃): 0.934
3. relative vapor density (g/ml, air=1): undetermined
4. melting point (ºc): 30-31
5. boiling point (ºc, normal pressure): 165
6. boiling point (ºc, 1.33kpa): 67.4
7. refractive index (n20): 1.448
8. flash point (ºc): 67
9. specific rotation degree (º): undetermined
10. autoignition point or ignition temperature (ºc): undetermined
11. vapor pressure (kpa, 25ºc): undetermined
12. saturated vapor pressure (kpa, 60ºc): undetermined
13. heat of combustion (kj/mol): undetermined
14. critical temperature (ºc) : undetermined
15. critical pressure (kpa): undetermined
16. log value of oil-water (octanol/water) partition coefficient: undetermined
17. explosion upper limit (%, v/v): undetermined
18. explosion lower limit (%, v/v): undetermined
19. solubility: can be compared with miscible in water and soluble in alcohol.
toxicological data
acute toxicity data
rat oral ld50: 2900 mg/kg
mouse oral ld50: 2150 mg/kg
rabbit oral ldlo: 1mg/kg
other multiple dose data
rat oral tdlo: 44800 mg/kg/8w-c
rat inhalation tclo: 230 ug/m3/4h/13w-i
dog oral tdlo: 11340 mg/kg/28d-c
primate-monkey inhaled tclo: 6 mg/m3/ 89d-i
rodent-hamsterinhalation tclo: 100 mg/m3/4h/13w-i
ecological data
none
molecular structure data
1. molar refractive index: 25.61
2. molar volume (cm3/mol): 95.7
3. isotonic specific volume (90.2k ): 232.1
4. surface tension (dyne/cm): 34.4
5. polarizability (10-24cm3): 10.15
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): -0.8
2. number of hydrogen bond donors: 2
3. number of hydrogen bond acceptors: 2
p>
4. number of rotatable chemical bonds: 1
5. number of tautomers: none
6. topological molecule polar surface area 46.2
7. number of heavy atoms: 6
8. surface charge: 0
9. complexity: 42.8
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
1. irritating to eyes and skin.
storage method
stored in a cool, dry and dark place.
synthesis method
1. react 2-nitropropane with formaldehyde aqueous solution, and then concentrate it into an aqueous solution containing 70% 2-methyl-2-nitro-1-propanol. dilute to 30% with methanol. add to the autoclave, add 5% skeleton nickel by weight of nitro, and hydrogenate and reduce at 30°c and 267 atmospheric pressure for 6-8 hours. after distilling off methanol and most of the water under normal pressure, distill under reduced pressure to collect the 103°c (8.53-8.67kpa) fraction, which is 2-amino-2-methyl-1-propanol. based on 2-nitropropane, the yield is 70.5%.
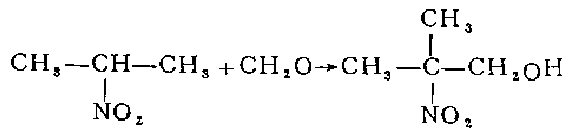
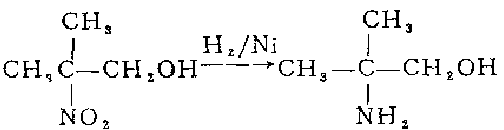
purpose
1. used to synthesize surfactants, vulcanization accelerators, and acid gas absorbers. derivatives formed with carboxylic acid compounds are used in gas chromatography analysis.
2.carbonyl protecting reagent. synthesize 2,2-dimethylaziridine (a very useful intermediate) with high yield. pine vinegar is the only highly efficient precipitant for l-piramic acid.
3. it is an organic base used as a neutralizing agent and is generally used as a neutralizing agent for resins in cosmetics.

 微信扫一扫打赏
微信扫一扫打赏

