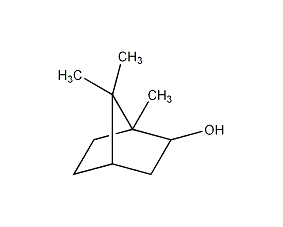
structural formula
| business number | 03jh |
|---|---|
| molecular formula | c10h18o |
| molecular weight | 154.25 |
| label |
bornhenol-2, isoborneol, isocampic acid-2, isoborneol, isobornyl alcohol, isobornyl alcohol, isocampool, 3-bornillol, dl-isoborneol, isoborneol, food additives, flavor enhancer |
numbering system
cas number:124-76-5
mdl number:mfcd00074821
einecs number:204-712-4
rtecs number:np7300000
brn number:none
pubchem number:24895930
physical property data
1. character: white or slightly yellow crystal
2. melting point: 212(seal)℃
3. optical rotation: left-handed [α]d+34.3°(ethanol); dextral [α]d-34.3°(ethanol)
4. boiling point (ºc): 214
toxicological data
skin/eye irritation data
rabbit skin contact: 500mg/24h moderate reaction
acute toxicity data
rat oral ld50: 5200mg/ kg
mouse vein ld50: 56mg/kg
rabbit skin ld50: >5mg/kg
ecological data
none
molecular structure data
5. molecular property data:
1. molar refractive index: 45.85
2. molar volume (cm3/mol): 155.3
3. isotonic specific volume (90.2k): 381.3
4. surface tension (dyne/cm): 36.2
5. polarizability (10-24cm3): 108.17
compute chemical data
1. hydrophobic parameter calculation reference value (xlogp): 2.7
2. number of hydrogen bond donors: 1
3. number of hydrogen bond acceptors: 1
4. number of rotatable chemical bonds: 0
5. number of tautomers:
6. topological molecular polar surface area (tpsa): 20.2
7. number of heavy atoms: 11
8. surface charge: 0
9. complexity: 185
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 3
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters number: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
basic properties: bicyclic monoterpenoids, which are the main isomers of bohenol. sex��similar to camphor.
storage method
storage: store at room temperature.
synthesis method
none
purpose
usage: used as a spice in medicine and daily chemical products, and also as a preservative.

 微信扫一扫打赏
微信扫一扫打赏

