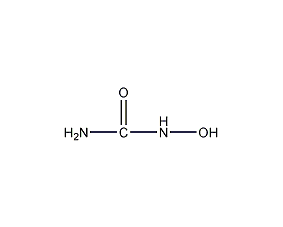
structural formula
| business number | 03kl |
|---|---|
| molecular formula | ch4n2o2 |
| molecular weight | 76.06 |
| label |
carbamylhydroxylamine, hydroxyurea, n-hydroxyurea, carbamoylhydroxylamine, aliphatic compounds |
numbering system
cas number:127-07-1
mdl number:mfcd00007943
einecs number:204-821-7
rtecs number:yt4900000
brn number:1741548
pubchem number:24278474
physical property data
1. character:white needle crystal
2. melting point (℃):142-146
3. water solubility:soluble
toxicological data
none
ecological data
none
molecular structure data
5. molecular property data:
1. molar refractive index: 15.37
2. molar volume (m3/mol):52.1
3. isotonic specific volume (90.2k):150.7
4. surface tension (dyne/cm):69.7
5. polarizability(10-24cm3):6.09
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 3
3. number of hydrogen bond acceptors: 2
4. number of rotatable chemical bonds: 0
5. number of tautomers: 4
6. topological molecule polar surface area 75.4
7. number of heavy atoms: 5
8. surface charge: 0
9. complexity: 42.9
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
basic properties
white needle-like crystals. melting point: 70-72℃ (decomposition) or 141℃ decomposition. easily soluble in water and hot ethanol, slightly soluble in cold ethanol, insoluble in ether; benzene. not stable in contact with water or heat. odorless and tasteless
storage method
storage
stored sealed in a cool, dry place.
synthesis method
brief description of production methods
obtained from the reaction of ethyl carbamate and hydroxylamine hydrochloride. cool the sodium hydroxide solution to 20-25°c. add ethyl carbamate and hydroxylamine hydrochloride alternately while stirring, and react at 25-28°c for 16 hours. neutralize with hydrochloric acid to ph 6.5-7, and control the temperature not to exceed 25°c. then concentrate under reduced pressure, filter while hot, and cool to below 0°c overnight to precipitate crystals. filter, wash the crystals with ice water, and dry to obtain crude hydroxyurea. the yield is about 65%. after refining, pharmaceutical grade hydroxyurea can be obtained.
purpose
purpose
this product is an anti-tumor drug.
y: arial; mso-ascii-font-family: ‘times new roman’; mso-hansi-font-family: ‘times new roman’; mso-bidi-font-family: arial”>(10-24cm3): 6.09
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 3
3. number of hydrogen bond acceptors: 2
4. number of rotatable chemical bonds: 0
5. number of tautomers: 4
6. topological molecule polar surface area 75.4
7. number of heavy atoms: 5
8. surface charge: 0
9. complexity: 42.9
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
basic properties
white needle-like crystals. melting point: 70-72℃ (decomposition) or 141℃ decomposition. easily soluble in water and hot ethanol, slightly soluble in cold ethanol, insoluble in ether; benzene. not stable in contact with water or heat. odorless and tasteless
storage method
storage
stored sealed in a cool, dry place.
synthesis method
brief description of production methods
obtained from the reaction of ethyl carbamate and hydroxylamine hydrochloride. cool the sodium hydroxide solution to 20-25°c. add ethyl carbamate and hydroxylamine hydrochloride alternately while stirring, and react at 25-28°c for 16 hours. neutralize with hydrochloric acid to ph 6.5-7, and control the temperature not to exceed 25°c. then concentrate under reduced pressure, filter while hot, and cool to below 0°c overnight to precipitate crystals. filter, wash the crystals with ice water, and dry to obtain crude hydroxyurea. the yield is about 65%. after refining, pharmaceutical grade hydroxyurea can be obtained.
purpose
purpose
this product is an anti-tumor drug.

 微信扫一扫打赏
微信扫一扫打赏

