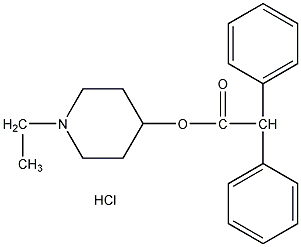
structural formula
| business number | 03m6 |
|---|---|
| molecular formula | c21h26clno2 |
| molecular weight | 359.90 |
| label |
diheperdone, aromatic compounds |
numbering system
cas number:129-77-1
mdl number:mfcd00057377
einecs number:204-964-5
rtecs number:none
brn number:none
pubchem id:none
physical property data
1. characteristics: colorless flammable liquid.
2. density (g/ml ,25/4℃): 0.7681
3. refractive index (n d20): 1.3900
4. flashpoint (℃):-15
5. melting point (℃):-112
6. boiling point (ºc):83
7. solubility:easily soluble in ethanol and ether, slightly soluble in water.
8. stability:chemically active, easy to undergo addition and polymerization reactions, easily hydrolyzed by acids into acetaldehyde and isobutanol.
toxicological data
1, acute toxicity: rat intravenously ld5o: 19mg/kg
mice orally ld5o: 1040mg/kg
mouse transvenous ld5o: 26mg/kg
dog meridian veinld5o:35mg/kg
ecological data
none yet
molecular structure data
none yet
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 1
3. number of hydrogen bond acceptors: 3
4. number of rotatable chemical bonds: 6
5. number of tautomers: none
6. topological molecule polar surface area 29.5
7. number of heavy atoms: 25
8. surface charge: 0
9. complexity: 367
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 1
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 2
properties and stability
none yet
storage method
none yet
synthesis method
introduction to production methods
produced by the base-catalyzed addition of acetylene and isobutanol.
purpose
usage
used as polymer monomer and organic synthesis intermediate.
amily: times new roman; mso-list: ignore”>8. stability:reactive chemical properties, prone to addition and polymerization reactions, and easily hydrolyzed by acids into acetaldehyde and isobutanol.
toxicological data
1, acute toxicity: rat intravenously ld5o: 19mg/kg
mice orally ld5o: 1040mg/kg
mouse transvenous ld5o: 26mg/kg
dog meridian veinld5o:35mg/kg
ecological data
none yet
molecular structure data
none yet
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 1
3. number of hydrogen bond acceptors: 3
4. number of rotatable chemical bonds: 6
5. number of tautomers: none
6. topological molecule polar surface area 29.5
7. number of heavy atoms: 25
8. surface charge: 0
9. complexity: 367
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 1
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 2
properties and stability
none yet
storage method
none yet
synthesis method
introduction to production methods
produced by the base-catalyzed addition of acetylene and isobutanol.
purpose
usage
used as polymer monomer and organic synthesis intermediate.

 微信扫一扫打赏
微信扫一扫打赏

