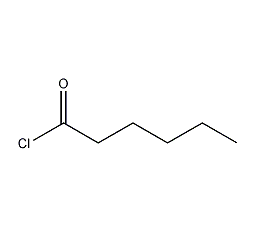
structural formula
| business number | 03v5 |
|---|---|
| molecular formula | c6h11clo |
| molecular weight | 134.60 |
| label |
crystalline calcium oxide, caproyl chloride |
numbering system
cas number:142-61-0
mdl number:mfcd00000760
einecs number:205-549-1
rtecs number:none
brn number:506332
pubchem number:24849650
physical property data
1. physical property data:
1.character: colorless or light yellow liquid
2.relative density: 0.9805
3.refractive index: 1.4286
4.flash point (℃):50
5.melting point (℃): -87.3
6.boiling point (ºc): 151-153
7.solubility: soluble in most organic solvents such as ether and chloroform.
toxicological data
none
ecological data
3. ecology data:
1, other harmful effects: this substance may be harmful to the environment, special attention should be paid to water bodies.
molecular structure data
5. molecular property data:
1. molar refractive index:34.72
2. molar volume (cm3/mol): 136.0
3. isotonic specific volume (90.2k ):314.3
4. surface tension (dyne/ cm):28.4
5. polarizability(10-24cm3):13.76
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 0
3. number of hydrogen bond acceptors: 1
4. number of rotatable chemical bonds: 4
5. number of tautomers: none
6. topological molecule polar surface area 17.1
7. number of heavy atoms: 8
8. surface charge: 0
9. complexity: 70.9
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
stable under normal temperature and pressure.
incompatible materials:water, alcohols, strong oxidants, strong bases.
storage method
store sealed in a cool and dry place.
synthesis method
obtained from the reaction of n-ethanol and thionyl chloride. add thionyl chloride to 50-70℃ mixed with n-hexanoic acid at 70-90℃ reaction 4h. distilled, collected147-160℃ fraction to obtain hexanoyl chloride.
purpose
1.this product is used as an intermediate in organic synthesis. used to prepare the antifungal drug tribromophenyl hexanoate and also used as a liquid crystal intermediate.
2.for organic synthesis.
3.used as an acylating agent in organic synthesis.
t-size: 9pt; font-family: 宋体; mso-bidi-font-family: arial; mso-ascii-font-family: arial; mso-hansi-font-family: arial”>polarizability (10-24 cm3):13.76
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 0
3. number of hydrogen bond acceptors: 1
4. number of rotatable chemical bonds: 4
5. number of tautomers: none
6. topological molecule polar surface area 17.1
7. number of heavy atoms: 8
8. surface charge: 0
9. complexity: 70.9
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
stable under normal temperature and pressure.
incompatible materials:water, alcohols, strong oxidants, strong bases.
storage method
store sealed in a cool and dry place.
synthesis method
obtained from the reaction of n-ethanol and thionyl chloride. add thionyl chloride to 50-70℃ mixed with n-hexanoic acid at 70-90℃ reaction 4h. distilled, collected147-160℃ fraction to obtain hexanoyl chloride.
purpose
1.this product is used as an intermediate in organic synthesis. used to prepare the antifungal drug tribromophenyl hexanoate and also used as a liquid crystal intermediate.
2.for organic synthesis.
3.used as an acylating agent in organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

