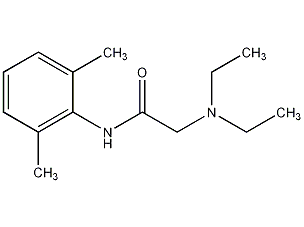
structural formula
| business number | 03rp |
|---|---|
| molecular formula | c14h22n2o |
| molecular weight | 234.34 |
| label |
n-diethylaminoacetyl-2,6-dimethylaniline, xylocaine, xylocaine, lidocaine base, n-diethylacetyl-2,6-dimethylaniline, 2-diethylamino-n-(2,6-dimethylphenyl)acetamide, 2-diethylamino-n-(2,6-dimethylphenyl)acetamide, lignocaine, xylocaine, aromatic compounds |
numbering system
cas number:137-58-6
mdl number:mfcd00026733
einecs number:205-302-8
rtecs number:an7525000
brn number:none
pubchem number:24896480
physical property data
1. appearance: white crystalline powder
2. melting point (℃): 76~79
3. solubility: easily soluble in water or ethanol, soluble in chloroform , insoluble in ether.
toxicological data
none
ecological data
none
molecular structure data
1. molar refractive index: 72.42
2. molar volume (cm3/mol): 228.3
3. isotonic specific volume (90.2k): 571.1
4. surface tension (3.0 dyne/cm): 39.1
5. polarizability (0.5 10-24cm3): 28.71
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 1
3. number of hydrogen bond acceptors: 2
4. number of rotatable chemical bonds: 5
5. number of tautomers: 3
6. topological molecule polar surface area 32.3
7. number of heavy atoms: 17
8. surface charge: 0
9. complexity: 228
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
none
storage method
this product should be sealed and stored in a cool, dry place.
synthesis method
1. chloroacetyl chloride method
2,6-dimethylaniline undergoes chloroacetylation with chloroacetyl chloride, then condenses with diethylamine, and then acidifies to form a salt to obtain lidocaine.
2. diethylaminoacetic acid methyl ester method
in the presence of sodium methoxide, 2,6-dimethylaniline and n,n-diethylaminoacetic acid methyl ester are condensed, it is then acidified to form a salt to obtain the product.
purpose
widely used for topical anesthesia, infiltration anesthesia, conduction anesthesia, and epidural anesthesia. it is also an important drug in the treatment of sudden arrhythmias.

 微信扫一扫打赏
微信扫一扫打赏

