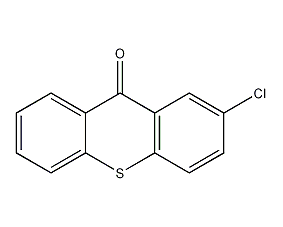
structural formula
| business number | 01wm |
|---|---|
| molecular formula | c13h7clos |
| molecular weight | 246.71 |
| label |
2-chlorothioxanth-9-one, 2-chlorothioxanthen-9-one, 9h-thioxanthen-9-one |
numbering system
cas number:86-39-5
mdl number:mfcd00005067
einecs number:201-667-2
rtecs number:none
brn number:none
pubchem number:24892945
physical property data
1. characteristics: yellow crystal.
2. density (g/ml,25/4℃): undetermined
3. relative vapor density (g/ml, air=1): undetermined
4. melting point (ºc):152.5-153.5 °c
5. boiling point (ºc,normal pressure): undetermined
6. boiling point (ºc,5.2kpa): undetermined
7. refractive index: undetermined
8. flashpoint (ºc):196 °f
9. specific optical rotation (º): undetermined
10. autoignition point or ignition temperature (ºc): undetermined
11. vapor pressure (kpa,25ºc): undetermined
12. saturated vapor pressure (kpa,60ºc3. isotonic ratio (90.2k): 479.1
4. surface tension (dyne/cm): 57.5
5. polarizability(10-24 cm3):26.55
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 0
3. number of hydrogen bond acceptors: 2
4. number of rotatable chemical bonds: 0
5. number of tautomers: none
6. topological molecule polar surface area 42.4
7. number of heavy atoms: 16
8. surface charge: 0
9. complexity: 294
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
none yet
storage method
this product should be sealed and stored in a cool and dry place.
synthesis method
it is obtained from anthranilic acid through diazotization, condensation and ring closure. first mix anthranilic acid, hydrochloric acid, and sodium nitrite at 0-5℃carry out diazotization reaction to obtain a diazonium salt solution; then perform a condensation reaction with an alkali solution of p-chlorothiophenol, and acidify with hydrochloric acid to obtain4-chlorodiphenyl sulfide-2′carboxylic acid, mix it with concentrated sulfuric acid in60-70℃catalytic closed loop to obtain the finished product.
purpose
pharmaceutical intermediate, used in the synthesis of the tranquillizer telden (chlorprothixene,[113-59-7]).
ning: 0pt”>carry out diazotization reaction to obtain a diazonium salt solution; then perform a condensation reaction with an alkali solution of p-chlorothiophenol, and acidify with hydrochloric acid to obtain4-chlorodiphenyl sulfide-2′carboxylic acid, mix it with concentrated sulfuric acid60-70℃catalytic closed loop to obtain the finished product.
purpose
pharmaceutical intermediate, used in the synthesis of the tranquillizer telden (chlorprothixene,[113-59-7]).

 微信扫一扫打赏
微信扫一扫打赏

