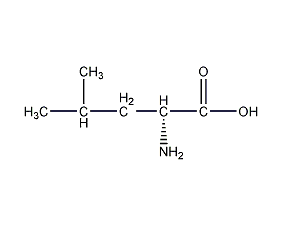
structural formula
| business number | 0482 |
|---|---|
| molecular formula | c6h13no2 |
| molecular weight | 131.17 |
| label |
d-leucine, d-2-amino-4-methylpentanoic acid, d-leucine (d-leucine), d-leucine, (r)-(-)-leucine, (r)-leucine, (r)-2-amino-4-methylpentanoic acid, (r)-2-amino-4-methylvaleric acid, d-leucin, leucine, d-, d-leu, d-leucine |
numbering system
cas number:328-38-1
mdl number:mfcd00063088
einecs number:206-327-7
rtecs number:oh2840000
brn number:1721721
pubchem number:24874859
physical property data
一 , physical property data
traits :white flaky crystals.
density (g/ml,25/4℃): not available
relative vapor density (g/ml, air=1):not available
melting point (ºc):293
boiling point (ºc, normal pressure): not available
boiling point (ºc, 5.2kpa): not available
refraction rate: not available
flash point (ºc): not available
optical rotation (º): 10.34
spontaneous combustion point or ignition temperature (ºc): not available
steam pressure (kpa, 25ºc): not available
saturated vapor pressure (kpa, 60ºc): not available
burn heat (kj/mol):not available
critical temperature (ºc): not available
critical pressure (kpa): not available
oil and water log value of the (octanol/water) partition coefficient:not available
explosion upper limit (%, v/v): not available
explosion lower limit (%, v/v): not available
dissolve sex: soluble in water
toxicological data
two , toxicological data:
acute toxicity:mid lethal dose (rat, intraperitoneal)642mg/kg. .
ecological data
three , ecological data:
1 ,other harmful effects: this substance may be harmful to the environment, and special treatment should be given to water bodies. notice.
molecular structure data
1. molar refractive index: 34.86
2. molar volume (m3/mol):126.6
3. isotonic specific volume (90.2k):316.4solubility :soluble in water
toxicological data
two , toxicological data:
acute toxicity:mid lethal dose (rat, intraperitoneal)642mg/kg. .
ecological data
three , ecological data:
1 ,other harmful effects: this substance may be harmful to the environment, and special treatment should be given to water bodies. notice.
molecular structure data
1. molar refractive index: 34.86
2. molar volume (m3/mol):126.6
3. isotonic specific volume (90.2k):316.4
4. surface tension (dyne/cm):39.0
5. polarizability(10-24cm3):13.82
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 2
3. number of hydrogen bond acceptors: 3
4. number of rotatable chemical bonds: 3
5. number of tautomers: none
6. topological molecule polar surface area 63.3
7. number of heavy atoms: 9
8. surface charge: 0
9. complexity: 101
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 1
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
none yet
storage method
should be sealed and stored dry.
synthesis method
use acetyl-dl-leucine as raw material, remove l-leucine through acylase treatment, then hydrolyze with hydrochloric acid to obtain crude product, and obtain pure product after crystallization and purification.
purpose
for biochemical research.
>
4. surface tension (dyne/cm):39.0
5. polarizability(10-24cm3):13.82
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 2
3. number of hydrogen bond acceptors: 3
4. number of rotatable chemical bonds: 3
5. number of tautomers: none
6. topological molecule polar surface area 63.3
7. number of heavy atoms: 9
8. surface charge: 0
9. complexity: 101
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 1
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
none yet
storage method
should be sealed and stored dry.
synthesis method
use acetyl-dl-leucine as raw material, remove l-leucine through acylase treatment, then hydrolyze with hydrochloric acid to obtain crude product, and obtain pure product after crystallization and purification.
purpose
for biochemical research.

 微信扫一扫打赏
微信扫一扫打赏

