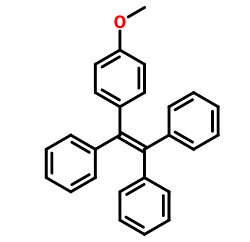
background and overview[1]
after hydrolysis of 1-(4-methoxyphenyl)-1,2,2-tristyrene, the intermediate 1-(4-hydroxyphenyl)-1,2,2-tristyrene can be prepared. the intermediate can be used to synthesize tetrastyrene schiff base red light zinc ion probe to effectively detect atypical metal ions.
preparation[1]
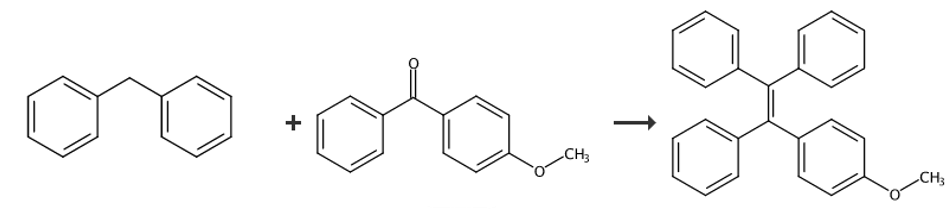
dissolve diphenylmethane (12mmol, 2.02g) in 50ml tetrahydrofuran, at minus 20°c, under nitrogen protection, add dropwise 2.5m n-butyllithium (10mmol, 4ml), and then add dropwise 4-methoxybis benzophenone (12mmol, 3.03g) was heated to room temperature, stirred for 3 hours, quenched with water, extracted with dichloromethane, spin off the solvent, added toluene, p-toluenesulfonic acid (1.8mmol, 0.342g), refluxed for 6 hours, and cooled at room temperature, wash twice with 5% sodium bicarbonate aqueous solution, dry over anhydrous magnesium sulfate, spin off the solvent, and obtain a yellow crude product, which is recrystallized to obtain a white solid product compound 1-(4-methoxyphenyl)-1,2. 2-tristyrene, yield 80%. 1hnmr (400mhz, dmso-d6) δ (tms, ppm): 7.19-7.06 (m, 9h), 7.03-6.92 (m, 6h), 6.87 (d, j=8.7hz, 2h), 6.70 (d, j=8.8hz, 2h), 3.67 (s, 3h).
apply[1]
the intermediate 1-(4-hydroxyphenyl)-1,2,2-tristyrene can be prepared after hydrolysis of 1-(4-methoxyphenyl)-1,2,2-tristyrene. to synthesize tetrastyrene schiff base red light zinc ion probe, the specific reaction steps are as follows: compound 1-(4-methoxyphenyl)-1,2,2-tristyrene (4.4mmol, 1.72g) is dissolved in glacial acetic acid, add 48% hydrobromic acid (50ml), reflux and stir for 12 hours, evaporate the solvent, add toluene and re-evaporate, extract with ethyl acetate, wash twice with sodium bicarbonate and saturated brine, and dry over anhydrous magnesium sulfate , spin off the solvent, and perform column chromatography (n-hexane/ethyl acetate = 10:1) to obtain the product 1-(4-hydroxyphenyl)-1,2,2-tristyrene. 1hnmr (400mhz, cdcl3)δ (tms, ppm) 7.16–7.10 (m, 9h), 7.08–7.02 (m, 6h), 6.92 (d, j=8.3hz, 2h), 6.59 (d, j=8.3hz, 2h), 4.61 (s, 1h).
references
[1]cn201710135796.5 tetrastyrene schiff base red light zinc ion probe and its preparation method and use

 微信扫一扫打赏
微信扫一扫打赏

