background and overview[1-2]
2,6,3′,5′-tetrahydroxystilbene is also called stilbene alcohol. methyl alcohol is a stilbene monomer compound isolated from the genus methyl. pharmacological experiments show that it has strong anti-inflammatory and antioxidant activities.
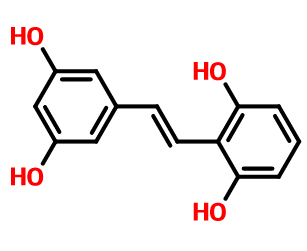
extraction method[1]
extract 10 kg of the cane stems of a. microphylla with 80% ethanol three times, recover the solvent under reduced pressure to obtain an ethanol extract, disperse it with hot water, and extract it with petroleum ether, ethyl acetate, and n-butanol in sequence. using a variety of column chromatography and thin layer preparative chromatography methods, compounds i (20 mg), ii (30 mg), iii (8 mg); iv (15 mg), v (20 mg) were separated from the ethyl acetate fraction. mg), vi (20 mg), vii (400 mg), viii (100 mg), ix (33 mg), and compound x (150 mg) was separated from n-butanol. ⅸ is 2,6,3′,5′-tetrahydroxystilbene.
apply[2]
2,6,3′,5′-tetrahydroxystilbene can be used to prepare 4-(6,8-dimethoxy-2-naphthyl)-1,3-benzenediol. 2,6,3′,5′-tetrahydroxystilbene is a new type of phenylnaphthalene derivative produced by losing two benzene rings from the dimer of 2,6,3′,5′-tetrahydroxystilbene. pharmacology activity test results showed that 4-(6,8-dimethoxy-2-naphthyl)-1,3-benzenediol showed strong antioxidant activity. the preparation method is as follows:
2,6,3′,5′-tetrahydroxystilbene (259 mg) was dissolved in 40 ml 5% h2so4 methanol solution, heat to reflux with stirring for 48 h. stop the reaction, add 150 ml of water to the reaction mixture, extract with etoac (50 ml×3), combine the organic layers, dry with na2so4, and concentrate under reduced pressure. silica gel column chromatography of the paste, chcl3-meoh (v:v=500:1) was eluted to obtain 4-(6,8-dimethoxy-2-naphthyl)-1 , 3-phenylenediol (60 mg, 38.2%), light black amorphous powder, m.p. 162~164 ℃; uv-vis (meoh) λmax: 224, 257,304 nm; ir (kbr)νmax: 3370, 2935, 2827 , 1699, 1628, 1601, 1500, 1454,1402, 1342, 1286, 1207, 1159, 1111, 1045, 997, 926, 833,696, 671 cm ): 296 (m+, 100), 185(10), 132 (17), 93 (24), 69 (14). hr-fab-ms calcd for c18h16o4 296.1049, found 296.1088.
references
[1]wang jianwei, liang jingyu, li li. chemical composition of m. lobata[j]. chinese natural medicines, 2006(06):432-434.
[2] yao chunsuo, lin mao, yang qingyun. preparation of active dimer derivatives of memantyl alcohol by oxidative coupling reaction and acid-catalyzed reaction [j]. organic chemistry, 2013, 33(02): 312- 318.

 微信扫一扫打赏
微信扫一扫打赏

