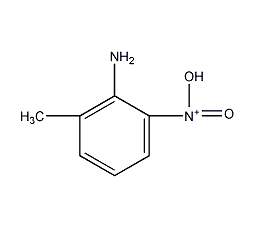
structural formula
| business number | 05u0 |
|---|---|
| molecular formula | c7h8n2o2 |
| molecular weight | 152.15 |
| label |
2-amino-3-nitrotoluene, 6-nitro-2-methylaniline, 6-nitro-o-toluidine, 6-nitro-o-toluidine, 2-amino-3-nitrotoluene, 6-nitro-o-toluidine, medicine |
numbering system
cas number:570-24-1
mdl number:mfcd00007744
einecs number:209-329-6
rtecs number:none
brn number:1868029
pubchem number:24869568
physical property data
1. physical property data
1. flashpoint (℃):110
2. melting point (℃):93-96
3. solubility:water-soluble<0.1 g/100 ml at 23°c
toxicological data
none
ecological data
none
molecular structure data
5. molecular property data:
1. molar refractive index: 41.85
2. molar volume (m3/mol):119.8
3. isotonic specific volume (90.2k):326.2
4. surface tension ():54.9
5. polarizability(10-24cm3):16.59
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): 2
2. number of hydrogen bond donors: 1
3. number of hydrogen bond acceptors: 3
4. number of rotatable chemical bonds: 0
5. number of tautomers: none
6. topological molecule polar surface area 71.8
7. number of heavy atoms: 11
8. surface charge: 0
9. complexity: 155
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
properties:
orange or yellow prismatic crystals. melting point97℃. soluble in alcohol, ether and benzene
and chloroform, slightly soluble in water.
storage method
none
synthesis method
for o-methylacetanilide70%nitro-o-methyl obtained by nitration with nitric acid
acetanilide, heated to boiling with concentrated hydrochloric acid, the reaction product is carried out
steam distillation to obtain6-nitro-o-toluidine, yield about50%.
preparation method:
purpose
uses: used in organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

