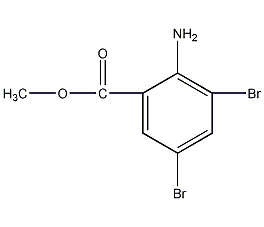
structural formula
| business number | 068y |
|---|---|
| molecular formula | c8h7br2no2 |
| molecular weight | 308.95 |
| label |
3,5-dibromo-2-aminobenzoic acid methyl ester, 3,5-dibromoanthranilic acid methyl ester, methyl 3,5-dibromoanthranilate, br2c6h2-2-(nh2)co2ch3 |
numbering system
cas number:606-00-8
mdl number:mfcd00010873
einecs number:none
rtecs number:none
brn number:none
pubchem number:24857930
physical property data
physical property data:
1. character:solid crystal
2. melting point (℃): 89~91
toxicological data
none
ecological data
3. ecological data:
1. other harmful effects: this substance may be harmful to the environment and should be harmful to water bodies. give special attention.
molecular structure data
5. molecular property data:
1, molar refractive index:57.64
2, molar volume (m3/mol):161.9
3, isotonic specific volume (90.2k ):438.9
4, surface tension (dyne/ cm):53.9
5、 polarizability (10-24cm3): 22.85
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): 3
2. number of hydrogen bond donors: 1
3. number of hydrogen bond acceptors: 3
4. number of rotatable chemical bonds: 2
5. number of tautomers: 3
6. topological molecule polar surface area 52.3
7. number of heavy atoms: 13
8. surface charge: 0
9. complexity: 201
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
1. basic properties
this product is a solid crystal. 98%the melting point of industrial products is89-91℃.
storage method
storage:
seal the secret container and store it in a sealed main container in a cool place dry position.
synthesis method
2. brief description of production method
obtained from the bromination of methyl anthranilate. combine methyl anthranilate with15%‘s hydrochloric acid stirring mix and cool until5-15℃, add bromine dropwise –hydrochloric acid mixture. gabby, in15℃the following reaction1h, adjust with sodium hydroxide solutionphthe value is2. filter, wash with water until neutral, and dry at low temperature to obtain the finished product. the yield is90%above.
purpose
3. purpose
for the synthesis of the drug bromide.

 微信扫一扫打赏
微信扫一扫打赏

