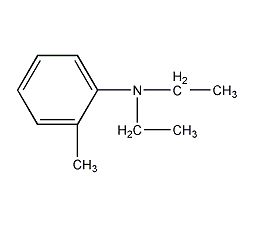
structural formula
| business number | 069c |
|---|---|
| molecular formula | c11h17n |
| molecular weight | 163.26 |
| label |
2-(diethylamino)toluene, 2-methyl-n,n-diethylaniline, n,n-diethyl o-methylaniline, (diethylamine)toluene |
numbering system
cas number:606-46-2
mdl number:none
einecs number:210-119-1
rtecs number:none
brn number:none
pubchem id:none
physical property data
physical property data:
1. character:colorless or light yellow oily liquid.
2. boiling point (ºc,100.7kpa):208-209
3. solubility:soluble in ethanol and ether, insoluble in water.
4. toxicity:poisonous.
toxicological data
none yet
ecological data
3. ecological data:
1. other harmful effects: this substance may be harmful to the environment and should be harmful to water bodies. give special attention.
molecular structure data
5. molecular property data:
1, molar refractive index:54.65
2, molar volume (m3/mol): 176.7
3, isotonic specific volume (90.2k ):426.5
4, surface tension (dyne/ cm):33.9
5、 polarizability (10-24cm3): 21.66
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 0
3. number of hydrogen bond acceptors: 1
4. number of rotatable chemical bonds: 3
5. number of tautomers: none
6. topological molecule polar surface area 3.2
7. number of heavy atoms: 12
8. surface charge: 0
9. complexity: 118
10. number of isotope atoms: 0
11. determined number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13.determined number of stereocenters of chemical bonds: 0
14. number of uncertain stereocenters of chemical bonds: 0
15. number of covalent bond units: 1
properties and stability
properties and stability:
no decomposition products may occur under normal temperatures and pressures.
storage method
1. storage
should be sealed in a cool place store in light place.
synthesis method
2. brief description of production method
from o-toluidine and ethyl bromide derived from the reaction of alkanes.
purpose
3. purpose
used in organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

