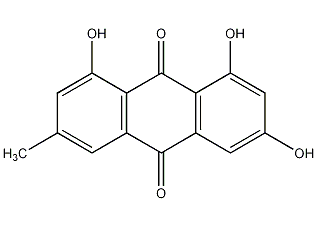
structural formula
| business number | 05c9 |
|---|---|
| molecular formula | c15h10o5 |
| molecular weight | 270.24 |
| label |
1,3,8-trihydroxy-6-methylanthraquinone, frangula-emodin, 6-methyl-1,3,8-trihydroxyanthraquinone |
numbering system
cas number:518-82-1
mdl number:mfcd00001207
einecs number:208-258-8
rtecs number:cb7920600
brn number:1888141
pubchem id:none
physical property data
1. characteristics: orange needle-like crystal
2. density (g/ cm3,25/4): undetermined
3. relative vapor density (g/cm3,air=1): undetermined
4. melting point (ºc):256-257
5. boiling point (ºc,normal pressure): undetermined
6. boiling point (ºc,8kpa): undetermined
7. refractive index: undetermined
8. flash point (ºf): undetermined
9. specific optical rotation (º): undetermined
10. autoignition point or ignition temperature (ºc): undetermined
11. vapor pressure (kpa,25ºc): undetermined
12. saturated vapor pressure (kpa,60ºc): undetermined
13. heat of combustion (kj/mol): undetermined
14. critical temperature (ºc): undetermined
15. critical pressure (kpa): undetermined
16. oil and water (octanol/log value of water) partition coefficient: undetermined
17. explosion limit (%,v/v): undetermined
18. lower explosion limit (%,v/v): undetermined
19. solubility:soluble in ethanol, insoluble in water
toxicological data
1. acute toxicity: mice intraperitoneally ld50: 35mg/kg, causing gastrointestinal and other changes;
2. mutagenicity data: microorganismstestsystem mutation: bacteria–salmonella typhimurium:50ug/plate;
microorganismtestsystem mutation: bacteria – salmonella typhimurium: 2ug/plate;
non-programdna synthesistest system: rodent – rat liver: 10mg/l;
微 testtest system: rodent – mouse lymphocyte: 60 umol/l ;
form transformationtest system: rodent – mouse fibroblast: 3mg/l;
somatic mutations in mammalscellstest system: rodent – mouse mammary gland: 5mg/l;
somatic mutations in mammalscellstestsystem: rodents–mouse lymphocytes:55500 nmol/l;
微 testtestsystem: rodent – hamster embryo:13750 ug/l;
sister chromatidsexchangetestsystem: rodent–hamster ovary:5mg/l;
somatic mutations in mammalscellstestsystem: rodents–hamster lung:10mg/l;
ecological data
this substance may be harmful to the environment, and special attention should be paid to water bodies.
molecular structure data
1. molar refractive index:69.13
2. molar volume (m3/mol):170.6
3. isotonic specific volume (90.2k):518.7
4. surface tension (dyne/cm): 85.4
5. polarizability(10-24 cm3):27.40
compute chemical data
1. hydrophobic parameters calculate the reference value (xlogp):2.7
2. hydrogen bonding number of donors: 3
3. hydrogen bonding number of receptors: 5
4. rotatable number of chemical bonds: 0
5, number of tautomers:135
6. topological molecules polar surface area (tpsa):94.8
7. heavy atom quantity: 20
8. surface charge :0
9. complexity :434
10. isotope atomic number:0
11. determine the number of atomic stereocenters:0
12. uncertain number of atomic stereocenters:0
13. determine the number of stereocenters of chemical bonds:0
14. uncertain number of chemical bond stereocenters:0
15. number of covalent bond units: 1
properties and stability
orange needle-shaped crystal. soluble in caustic alkali aqueous solution and sodium carbonate aqueous solution, and appears cherry red in ammonia solution.
storage method
stored in a cool, dry, well-ventilated warehouse. keep away from fire and heat sources. protect from direct sunlight. the packaging is sealed. they should be stored separately from acids and food chemicals, and avoid mixed storage. suitable materials should be available in the storage area to contain spills.
synthesis method
emodin is widely found in plant-based laxatives, such as in rhubarb rhizomes, buckthorn bark and root bark, and cassia seeds. emodin can be extracted from rhubarb rhizomes. emodin can also be prepared by synthesis, for example as 2- methyl anthraquinone, or 3,5-emodin can be produced by using dinitrobenzene anhydride and m-cresol as raw materials.
purpose
this product can be used as a laxative. although emodin it has purgative activity, but because it is easily destroyed by oxidation in the body, its purgative effect is actually very weak. if it is combined with sugar to form glycosides, it can exert a purgative effect. emodin-1-o-β-d- glucoside and emodin-8-o-β-d-glucoside is the glycoside that combines emodin and glucose. the two are just in different binding positions and are present in rhubarb at the same time.

 微信扫一扫打赏
微信扫一扫打赏

