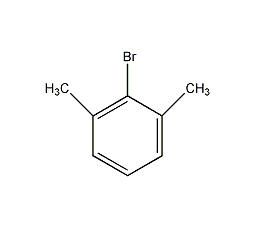
structural formula
| business number | 05v3 |
|---|---|
| molecular formula | c8h9br |
| molecular weight | 185.06 |
| label |
2-bromo-m-xylene, 2,6-dimethylbromobenzene, 2-bromo-1,3-dimethylbenzene, 2,6-dimethylphenylbromide, 2-bromo-1,3-dimethyl-benzen, aromatic compounds |
numbering system
cas number:576-22-7
mdl number:mfcd00000075
einecs number:209-397-7
rtecs number:cy9020000
brn number:1929780
pubchem number:24851276
physical property data
1. physical property data
1. density: 1.389g/ml at 25 °c (lit.
2. melting point (ºc): -10
3. boiling point (ºc, 5.2kpa): 206
4. refractive index: n20/d 1.554(lit.)
5. flash point (ºc): 73
toxicological data
none yet
ecological data
3. ecological data:
1. other harmful effects: this substance may be harmful to the environment, and special attention should be paid to water bodies.
molecular structure data
5. molecular property data:
1. molar refractive index: 43.59
2. molar volume (cm3/mol): 138.1
3. isotonic specific volume (90.2k): 333.0
4. surface tension (dyne/cm): 33.7
5. polarizability (10-24cm3): 17.28
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): 3.3
2. number of hydrogen bond donors: 0
3. number of hydrogen bond acceptors: 0
4. number of rotatable chemical bonds: 0
5. number of tautomers: none
6. topological molecule polar surface area 0
7. number of heavy atoms: 9
8. surface charge: 0
9. complexity: 80.6
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
stability and reactivity:
materials to avoid: oxides.
products to be decomposed: carbon monoxide and carbon dioxide, hydrogen bromide.
storage method
storage:
seal the container and store it in a sealed main container in a cool, dry place.
synthesis method
add 610g (5mol) 2,6-dimethylaniline and 3.75l hydrogen into a 5l beakerbromic acid, cool it under rapid stirring to form fine crystals, and use an ice-salt bath for external cooling. control the reaction temperature to be lower than 15°c, and remove the liquid surface from the liquid surface under stirring. slowly add a solution of 385g (5.5mol) sodium nitrite dissolved in 400ml water. gradually add this diazonium salt solution into a heated reactor with a reflux condenser; after the nitrogen evolution reaction has eased, incubate it in an 80°c water bath for 3 hours. steam distill or heat to evaporate the crude product, wash it three times with 10% naoh, wash with water, dry and fractionate under reduced pressure. collect the 80~84°c (1.7~2.0kpa) fraction to obtain 680g (73%) of the product. 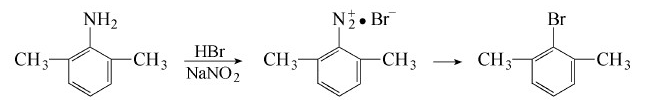
purpose
organic synthesis, pharmaceutical intermediates

 微信扫一扫打赏
微信扫一扫打赏

